
ʵ������ҪO��1 mol��L NaOH��Һ450 mL��0��5mol��l������Һ480 mL��������������Һ����������ش��������⣺
(1)��ͼ��ʾ��������������Һ��Ҫ���� (���ֺ�)������������Һ�����õ��IJ��������� (����������)��
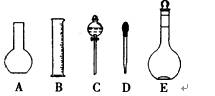
(2)���в����У�������ƿ���߱��Ĺ����� ��
A������һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������Ũ�Ⱥ�����ı���Һ
D��ϡ��ijһŨ�ȵ���Һ
E����ȡһ�������Һ��
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ�� (����ڡ������ڡ���С�ڡ�)O��1 mol��l����NaOH��Һ��ת��������ƿʱ������������������Ҫ��β����� ��(4)���ݼ����֪��������������Ϊ98�����ܶ�Ϊl.84 g��cm3��Ũ��������Ϊ ml�����ʵ������10 mL��15 mL��20 mL��Ͳ��Ӧѡ�� mL����Ͳ��á����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ����������
(5)���в�����ʹ���Ƶ�NaOH��ҺŨ��ƫ�� ���ǡ�
A������ֽ����NaOH
B��ѡ�õ�����ƿ������������ˮ
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶���
D���������ƹ����У�����ƿ����
��֪ʶ�㡿һ�����ʵ���Ũ����Һ�����ơ�
���𰸽�������1��BDE��2�֣����ձ��Ͳ�������2�֣�����2��A��1�֣�����3��2.0��1�֣������ڣ�1�֣����������ã�1�֣�����4��13.6��1�֣���15��1�֣�����Ũ�������ձ��ڱڻ�������ˮ�У����ò��������Ͻ��裨2�֣�����5��AC��2�֣�
��������1��������ƿ������Һ���ý�ͷ�ιܶ��ݣ���Ͳ��ȡˮ���ʴ�Ϊ��BDE������Ҫ�õ��������У��ܽ��ϡ��ҩƷ���ձ�������������õIJ�������
��2������ƿ�����ü�Ϊ������һ�����ȷŨ�ȵı���Һ����ֻ��A��ȷ��
��3��m=n*M=0.1mol/L*0.5L*40g/mol=2.0g��������ʱ���ӿ̶��ߣ�����Һ���ƫС����������ҺŨ��ƫ����NaOH��Һ��ת��������ƿʱ��������������ֻ������������Һ��
��4��Ũ�����Ũ��C=1000��w/M=1000*1.84*98%/98mol/L=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=0.5/L��0.5L��V=0.0136L=13.6mL��ѡȡ����Ͳ���Ӧ�õ��ڻ������ȡ��Һ�������ѡ15mL��Ͳ��ϡ��Ũ�������ȷ�����ǣ���Ũ�������ձ��ڱڻ�������ˮ�У����ò��������Ͻ��裬��ֹҺ�彦����
��5��A��NaOH���⣬����ֽ������ʹ����NaOH�������٣���Ũ��ƫ�ͣ�B��Ӱ�죻C��ˮ������Һ�������Ũ�Ƚ��ͣ�D���������õ���Һ����ȣ�ȡ��ʱ��ҺŨ�Ȼ�ƫ��ƫ�ͣ��ʴ�ΪAC
��˼·�㲦�����⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ����״����ǣ�2���⣬ע����Ҫ450mL��Һ����Ϊû��450mL������ƿ����500mL������ƿ������Ҫ����500mL��Һ���������������������ȡ��Һ�����Ӧ��������500mL��Һʱ���õ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NaOH��Һ�ζ�����ʵ���У������õ�����(����)
A����̪ B��Բ����ƿ
C����ƿ D����ʽ�ζ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)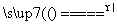 Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
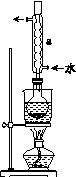
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O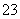 ��I2===S4O
��I2===S4O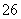 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO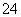 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�ѡ���е�������Ӧ,����ͬһ�����ӷ���ʽ��ʾ���� ( )
| ѡ�� | �� | �� |
| A | Ba(OH)2��Һ�����NaHCO3��Һ��� | NaOH��Һ�����NaHCO3��Һ��� |
| B | ����SO2ͨ��Ba(OH)2��Һ�� | ����SO2ͨ��Ba(OH)2��Һ�� |
| C | BaCl2��Һ��Na2SO3��Һ��� | Ba(OH)2��Һ��H2SO3��Һ��� |
| D | ������ˮ����AlCl3��Һ�� | ����AlCl3��Һ���백ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���û�з�����ѧ��Ӧ����
A���û���̿ȥ�������е���ζ
B�����ȼ�ˮ��������ϲ���������
C���ý��ݹ����������Һ�Ĺ���������ˮ��
D���ú��轺�����۵���С����ʳƷһ���ܷ��װ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧ��Ӧ����������ȷ����( )
A����Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ
B����֪NaOH(aq) +HCI(aq) =NaCI(aq)+H2O(1) ��H=-57.3 kJ��mol-1��
��40.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�57.3 kJ������
C��CO(g)��ȼ������283.0 kJ��mol-1�����ʾCO(g)��ȼ���ȵ��Ȼ�ѧ����ʽΪ
2CO(g)+O2(g)=2CO2(g) ��H=-283.0 kJ��mol-1
D����֪2C(s) +2O2(g)=2CO2(g) ��H=a��2C(s) +O2(g)=2CO(g) ��H=b����b>a
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
��1����ҵұ�����Ļ�ѧ����ʽ������������������������
��2����ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��
������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ��
�ٸõ��۵�������Ӧʽ������������������������
��ͨ�翪ʼ������������ҺpH�����������ԭ��������������������������
�۳�ȥ���ʺ������������Һ����Һ������������(��д��A����B��)������
��3�����ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)+10C(s) 6CaO(s)+P4(s)+10CO(g)����H1=+3 359��26 kJ��mol-1
6CaO(s)+P4(s)+10CO(g)����H1=+3 359��26 kJ��mol-1
CaO(s)+SiO2(s) CaSiO3(s)����H2=-89��61 kJ��mol-1
CaSiO3(s)����H2=-89��61 kJ��mol-1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s) 6CaSiO3(s)+P4(s)+10CO(g)����H3
6CaSiO3(s)+P4(s)+10CO(g)����H3
��H3=�������� kJ��mol-1��
 ��4�����ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
��4�����ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
2SO2(g)+O2(g) 2SO3(g)����H1=-197 kJ��mol-1��
2SO3(g)����H1=-197 kJ��mol-1��
H2O(g) H2O(l)����H2=-44 kJ��mol-1��
H2O(l)����H2=-44 kJ��mol-1��
2SO2(g)+O2(g)+2H2O (g) 2H2SO4(l)����H3=-545 kJ��mol-1��
2H2SO4(l)����H3=-545 kJ��mol-1��
��SO3(g)��H2O(l)��Ӧ���Ȼ�ѧ����ʽ��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���¶��£�������X������Y��0.16mol����10L�����ܱ������У�������Ӧ
X(g)��Y(g)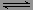 2Z(g) ��H��0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ����������
2Z(g) ��H��0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ����������
����
| t/min | 2 | 4 | 7 | 9 |
| n(Y)/mol | 0.12 | 0.11 | 0.10 | 0.10 |
����˵����ȷ����
A�����¶��´˷�Ӧ��ƽ�ⳣ��K��1.44
B�������������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰ��(��)����(��)
C����Ӧǰ2min��ƽ��������(Z)��2.0��10��3mol��L��1��min-1
D�������������䣬�ٳ���0.2mol Z��ƽ��ʱX�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6ƿ��ɫ�����Լ����ֱ����Ȼ������������ơ������ơ�����李���ˮ����ͭ��̼���ơ���ֻ�ṩ����ˮ��ͨ�������ʵ�鲽�輴�ɼ������ǡ�����д���пհף�
��1����ȡ���������Լ��ֱ����6֧�Թ��У�������������ˮ�����Թܣ��۲쵽�������ǣ� ��
����������ʵĻ�ѧʽ������ʽ����
��2���ֱ�ȡδ�������Һ�������м��������Ѽ������Һ���۲쵽���������Ӧ�����ӷ���ʽ�ǣ�
����������ʵĻ�ѧʽ������ʽ����
��3����������δ������ʵķ����۲쵽�������ǣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com