
 �ӻ�������DZ�ˮ���� �����С������
�ӻ�������DZ�ˮ���� �����С������
 ����ġ����ܡ����� kJ / mol ��
����ġ����ܡ����� kJ / mol ��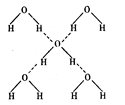
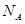 ��ʾ�����ӵ���������CaO�������Ϊ cm3��
��ʾ�����ӵ���������CaO�������Ϊ cm3��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO2��SO2��BF3����������ԭ�ӵ��������Ӷ�������8e���ȶ��ṹ |
| B��P4��CH4����������������Ҽ��Ƕ�Ϊ109o28�@ |
| C��CsCl��������ÿ��Cs+��������������Cl-����8�� |
| D��ԭ�Ӿ�����۷е�һ���Ƚ�������ĸ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


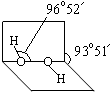
 R(OH)3
R(OH)3 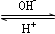 [R(OH)4]��
[R(OH)4]���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
��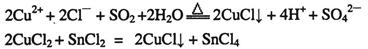
 ��
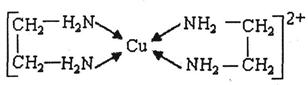
�� �е�ԭ�ӹ�����ӻ�����Ϊ ���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_ ��
�е�ԭ�ӹ�����ӻ�����Ϊ ���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_ ��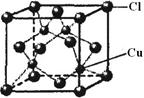
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ַ�������ԭ�ӵ��ӻ����Ͳ�ͬ |
| B�����ַ����м��ļ��Ժͷ��ӵļ��Բ�ͬ |
| C��NH3�����д�����һ��δ�ɼ��ŶԵ��� |
| D����������֮��ͼ������֮������������Ͳ�ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 (��ԭ�Ӽ�����������������ģ������ǻ����ʼ�����ȫ��ͬ)
(��ԭ�Ӽ�����������������ģ������ǻ����ʼ�����ȫ��ͬ) 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ȶ��ԣ�Na2CO3��CaCO3��NaHCO3 |
| B�����뾶��Fe(OH)3������K+��C1-��Na+ |
| C������������ӵ�������CH3COOH��C2H5OH��H2O |
| D���⻯��ķе㣺H2Se��H2S��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��CO2 | B��PCl3 | C��CCl4 | D��BF3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com