
������������ijЩ���⣬�����漰����ѧ֪ʶ�����и���������ȷ����
A�����ӵ��Ρ������߸�ţ�̡���������Ӫ��Ʒ����ʳ��Ʒ�еĵ⡢�ơ�����ָ����
B�����Ͻ�Ĵ���ʹ�ù鹦���������ý�̿�Ȼ�ԭ�����������л�ȡ������
C��ҽ���Ͻ���θ����Ӱǰ�����߷��õġ����͡���BaCO3�Ȳ�����ˮ������
D��������Գ��⡱�����ȵĴ�����Һȥ���ۡ����������˻�ѧ�仯
D
��������
���������A�����ӵ��Ρ������߸�ţ�̡���������Ӫ��Ʒ����ʳ��Ʒ�еĵ⡢�ơ�����ָԪ�أ�����B�����Ͻ�Ĵ���ʹ�ù鹦�������õ�ⷨ������ڵ��������л�ȡ�����ʣ�����C��ҽ���Ͻ���θ����Ӱǰ�����߷��õġ����͡���BaSO4�Ȳ�����ˮҲ��������������ʣ���ʹ��BaCO3���������ܹ������ᵼ���ؽ����ж�������D��������Գ��⡱�����ȵĴ�����Һȥ���ۡ������������µ����ʣ��������ǻ�ѧ�仯����ȷ��
���㣺���黯ѧ֪ʶ�������е�Ӧ�õ�֪ʶ��


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015����ʡ��ˮ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
����С��14�֣�ÿ��2�֣���ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���

��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��__ __��
��2��װ��B�б���ʳ��ˮ��������_________________________________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����__________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���___��
A | B | C | D | |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���� ����������װ��D��������Һ����װ��E�У����۲쵽��������_______________________________��
��5��ijͬѧ���齫װ��F�е�ҩƷ����������NaHSO3��Һ�������ȣ���ʦ��Ϊ���ף����ܷ�Ӧ�����ӷ���ʽ����ԭ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и�����ѧ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�˵���ͽ��۶���ȷ����
A����֪Cu2O + 2H+=Cu2+ + Cu + H2O��������ԭ����ͭ�����ú�ɫ��������ȫ����ϡ���ᣬ˵����ɫ������ͭ
B��������ˮ����̪��BaCl2��Һ����֪Ũ���������Һ���Լ����ɲⶨNaOH����(���ʽ�ΪNa2CO3)�Ĵ���
C����SO2����ͨ����ˮ�У���ˮ��ɫ����ȥ��˵��SO2����Ư����
D����֪Ksp(Ag2CrO4)=9.0��10-12, Ksp(AgCl)=1.8��10-10��˵��Ag2CrO4���ܽ��С��AgCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ������ѧ��12��������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼʾ���Ӧ�������������

A����ͼ1��ʾ���߿�֪����ѧ����ø����Ч����
B����H2S��Һ������ʵ���У���ͼ2��ʾ���߿�ȷ��ͨ�������XΪCl2
C����������������ʱ��2SO2(g)+ O2(g) 2SO3(g)ת����ϵ��ͼ3���У��������ʾO2��ת����
2SO3(g)ת����ϵ��ͼ3���У��������ʾO2��ת����
D��ͼ4����0.l000 mol��L��1������ζ�20.00 mL 0.l000mol��L��1 Na2CO3��Һ�����ߣ���a��b�㷴Ӧ�����ӷ���ʽΪ��HCO3����H�� = CO2����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ������ѧ��12��������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�ʵ����ȷ��
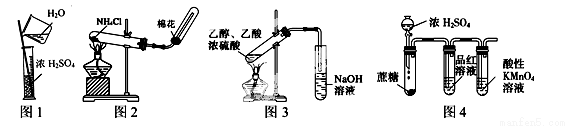
A��ͼ1��ϡ��Ũ����
B��ͼ2��ʵ�����Ʊ�����
C��ͼ3��ʵ�����Ʊ���������
D��ͼ4������Ũ���������Ƿ�Ӧ�����Ķ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ӱ�ʡ������ѧ�ڵڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
��15�֣�������̼����������ЧӦ������Ҫ���ʣ����ܼ��ţ���Ч������Դ���ܹ����ٶ�����̼���ŷš�
��1����һ���¶��µ�2L�̶��ݻ����ܱ������У�ͨ��2 molCO2��3mol H2�������ķ�ӦΪ��CO2(g)��3H2(g) ? CH3OH(g)��H2O(g)����H����a kJ��mol��1��a��0���� ���CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��

����˵���÷�Ӧ�Ѵ�ƽ��״̬����________����ѡ���ţ�
A��CO2����������ڻ�������б��ֲ���
B����������ƽ����Է�����������ʱ��ı仯���仯
C����λʱ����ÿ����1.2mol H2��ͬʱ����0.4molH2O
D������ϵ��H2O��CH3OH�����ʵ���Ũ��֮��Ϊ1:1���ұ��ֲ���
�ڼ�����¶��´˷�Ӧ��ƽ�ⳣ��K��_________����������λ��Ч���֣������ı����� ����ѡ�����ʹK��1��
A������ѹǿ
B������Ӧ��Ũ��
C�������¶�
D�������¶�
E���������
��2��ij�״�ȼ�ϵ��ԭ����ͼ1��ʾ��

��M��������Ӧ�ĵ缫��ӦʽΪ_______________________________��
���������������Դ����ͼ2װ�õ�ⱥ��ʳ��ˮ���缫��Ϊ���Ե缫������õ����ܷ�Ӧ���ӷ���ʽΪ�� ��������Һ���Ϊ300mL������Һ��pHֵ��Ϊ13ʱ���ڳ����²ⶨ�������������ļ״�������Ϊ______________��������Һ����仯����
��3����һ����CO2�����״�ȼ�ϵķ�����
��֪����CO2(g)��3H2(g)  CH3OH(g)��H2O(g) ��H����a kJ��mol��1��
CH3OH(g)��H2O(g) ��H����a kJ��mol��1��
��CH3OH(g)��CH3OH(l) ��H����b kJ��mol��1��
��2H2(g)��O2(g)��2H2O(g) ��H����c kJ��mol��1��
��H2O(g)��H2O(l) ��H����d kJ��mol��1��
���ʾCH3OH��l��ȼ���ȵ��Ȼ�ѧ����ʽΪ��_____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ӱ�ʡ������ѧ�ڵڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��ʾ��298 Kʱ��N2��H2��Ӧ�����������仯������ͼ ,���������������

A��������������ܸı�û�ѧ��Ӧ�ķ�Ӧ��
B��b�����Ǽ������ʱ�������仯����
C���÷�Ӧ���Ȼ�ѧ����ʽΪ��N2(g)��3H2(g)  2NH3(g)����H����92 kJ/mol
2NH3(g)����H����92 kJ/mol
D�����¶ȡ����һ���������£�ͨ��1 mol N2��3 molH2��Ӧ��ų�������Ϊ92kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ��ˮ�и���һ�ָ�ϰ����֪ʶ��⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣���ȩ�ڴ������ڵ������£����Ա��������������ᡣ���ݴ�ԭ�����ʵ���Ƶò����Թ�C���ռ�������������Һ����ͼ��ʾ���Թ�A��װ��40������ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ���ձ�B��װ��ijҺ�壩����֪��60�桫80��ʱ��˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���η�Ӧ������ȫ���й����ʵķе���±���
���� | ��ȩ | ���� | ���� | �Ҷ��� | ˮ |
�е� | 20.8�� | 117.9�� | 290�� | 197.2�� | 100�� |
��ش��������⣺
��1���Թ�A����60�桫80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������________________��
��2����ͼ��ʾ��ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�á���ʵ�鿪ʼʱ�¶ȼ�ˮ�����λ��Ӧ��___________��Ŀ����_____________________________�����Թ�A�ڵ���Ҫ��Ӧ��ɺ�Ӧ��������������¶ȼ�ˮ�����λ��Ӧ��___________________��

��3���ձ�B��ʢװ��Һ�������____________��д��һ�ּ��ɣ���
��4����������Թ�C���Ƿ��в������ᣬ���������ṩ��ҩƷ����Ʒ�У�����ʹ�õ���____________��������ĸ��
a��pH��ֽ b��̼�����Ʒ�ĩ
c����ɫʯ����ֽ d��������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015����ʡ��ԭ�и�һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ������
��15�֣��������й������ת���Ĺ�ϵͼ������A�׳����죬��Ϊǿ�ᣬ��Ϊ��ԭ�����壬��Ϊǿ����Һ��GΪ���ɫ������I����ɫ��dz��ɫ��

��1����F����Na����SO42-��ɵ���Һ����Ļ�ѧʽ�� _____________���о�A���ʵ�һ����;______________________________________________________________��
��2����D����ʹ����ʯ��ˮ����ǵ����壬���ҵĻ�ѧʽΪ________�������ڵ��ʡ��ᡢ���е�________����I��Һ�м�������������Һ�����Թ۲쵽��������______________________________��
��Ӧ�����ӷ���ʽ�ͻ�ѧ����ʽ������______________________��___________________��
��3��д��G��A��C�Ļ�ѧ����ʽ�� ___________________________________��
��4��д��E��C��Ӧ�ķ���ʽ���õ����ŷ��������ӵ�ת�Ʒ������Ŀ��__________��
��5����A�л�������Al2O3����ȥ���ʵķ����Ǽ��������________���÷�Ӧ�����ӷ���ʽΪ__________��
��6����E��A��ɵĻ������ϡH2SO4���ã�����ǡ���ܽ⣬������Һ�в���Fe3���������ɵ�Fe2����H2�����ʵ���֮��Ϊ4��1����Ӧ����A��E��H2SO4�����ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com