
| ���� | 1 | 2 | 3 | 4 |
| ��������������Һ��������g�� | 25 | 25 | 25 | 25 |
| ���ɳ�����������g�� | 2.9 | X | 8.7 | 8.7 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.005mol | B��0.01mol | C��0.025mol | D��0.03mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢ� | B���ڢۢ٢� | C���ܢڢ٢� | D���ۢ٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
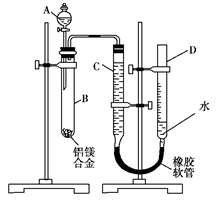
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������������������ | B����������Al(OH)3��Na2CO3�Ļ���� |
| C��1 mol NaAl(OH)2CO3��������3 mol H�� | D����ҩ�����ʺ���θ�����߷��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ǵؿ��ﺬ�����Ľ���Ԫ�� | B���ڳ����£������ܺ�������Ӧ |
| C������һ�ֱȽϻ��õĽ��� | D���ڻ�ѧ��Ӧ�У�������ʧȥ���ӣ��ǻ�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
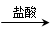 �����������������ml��������ɱ�״������
�����������������ml��������ɱ�״������ ���ʣ����������a�ˣ�
���ʣ����������a�ˣ�  ��Һ
��Һ ��ó���������b�ˡ�
��ó���������b�ˡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ�鲽�� | Ԥ��ʵ������ͽ��� |
| ����1������Ӧ������Һ���ˡ�ϴ�ӣ�ȡ�����������Թ��У�����������6mol��L-1 H2SO4��Һ����������á� | ___________________________________�� ֤����ɫ���庬��Si |
| ����2�� | |
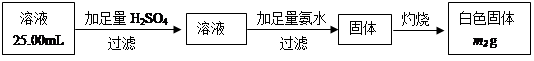
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
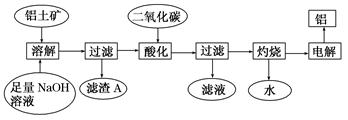
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com