
����Ŀ���Ķ�����֪ʶ���ش�1������2�����⡣
��.�ṹ��û�л�״�������������������������ʽ��CnH2n+2��nΪ����������������ÿ����2��̼�������Ȼͬʱ������1��̼̼�������������ؼ���˫������������Ҳ���������ӻ�״��������Ϊ������һ�������Ͷȣ���ϣ����ĸ����ʾ���ֳ���ȱ��ָ�����������ϩ����=1��������=4��
��.���л�������У���ij��̼ԭ����������4����ͬ��ԭ�ӻ�ԭ���ţ���̼ԭ�ӽв��Գ�̼ԭ�ӣ�ͨ�����Ǻű����*C��������������Ĺ�ѧ���ԡ�
��1���ݱ�������������״��˹��ϳ���һ���п������ԵĻ�����Depudecin���������������������������ṹ��ͼ��ʾ��
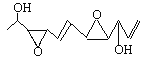
����д�����ֻ�����ķ���ʽ________________���û��������Ϊ____________��
��������ӵĽṹ����__________�����Գ�̼ԭ�ӣ�������Ŀ�и����Ľṹ��ʾ����*�����DZ�ʾ������
��2���½��ϳɵ�ij�л��ᆳ�ⶨ���й�ѧ���ԣ���ṹʽ��ͼ��ʾ��
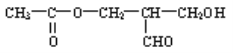
��Ҫʹ���л��ﲻ�پ��й�ѧ���ԣ���ɲ�����Щ��ѧ��������ֱ��г���__________________��__________________��__________________��д�����֣���
���𰸡�C11H16O446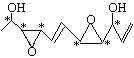 �ӳ���ȥ��ˮ�⡣
�ӳ���ȥ��ˮ�⡣
��������
��1���ٸ����л���̼ԭ�ӳɼ��ص㣬���л���ķ���ʽΪC11H16O4��������Hԭ��Ϊһ�������Ͷȣ��ó���һ����Ϊһ�������Ͷȣ�һ��˫��Ϊһ�������Ͷȣ����ݽṹ��ʽ�����л���IJ����Ͷ�Ϊ4���ڸ���������Ϣ������̼ԭ��Ϊ���Գ�̼ԭ�ӣ�����̼ԭ������4����ͬ��ԭ�ӻ�ԭ���ţ������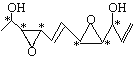 ����6�����Գ�̼ԭ�ӣ���2����������̼ԭ�ӵĶ��壬���У�CH2OH�ͣ�CHO�ġ�CH���е�CΪ����̼ԭ�ӣ����������ѧ���ԣ���Ҫ������H2�ļӳɷ�Ӧ������ˮ�ⷴӦ��������Ӧ����ȥ��Ӧ��
����6�����Գ�̼ԭ�ӣ���2����������̼ԭ�ӵĶ��壬���У�CH2OH�ͣ�CHO�ġ�CH���е�CΪ����̼ԭ�ӣ����������ѧ���ԣ���Ҫ������H2�ļӳɷ�Ӧ������ˮ�ⷴӦ��������Ӧ����ȥ��Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ϊȫ��������������������Ҫ���л��ܼ��������Dz��ֻ������������������ �ṹ��ʽ������ʽ��ijЩ�л�������ķ�Ӧʽ������ Pt��Ni �Ǵ�������



�ش��������⣺
��1����������____________��ͬ���칹�塣
��2���ӷ�Ӧ�١��ۿ��Կ������������������ӳɷ�Ӧ�Ļ�������____________�������ƣ����ж��� ��Ϊ____________��
��3����������������±�ص��ʣ�±���ⷢ�����ƵĿ����ӳɷ�Ӧ���绷������ HBr ��һ�������·� Ӧ���仯ѧ����ʽΪ____________������ע����Ӧ��������
��4��д��������ͱ�ϩ��һ�ַ������Լ�____________�����������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�鲻�ܴﵽʵ��Ŀ�Ļ���ʵ���������ȷ���� (����)
A. ���� B. �Ƚ���̼��������Ԫ�صķǽ�����
B. �Ƚ���̼��������Ԫ�صķǽ����� C. β������
C. β������ D. �ⶨ��ͭ��п�ĺ���
D. �ⶨ��ͭ��п�ĺ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)2017���п�Ժij�о��Ŷ�ͨ�����һ������Na-Fe3O4/HZSM-5��ܸ��ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ����ֵ���ͣ����о��ɹ�������Ϊ��CO2��ת�������ͻ���Խ�չ����
��֪��H2(g)+1/2O2(g)=H2O(l) ��H1 = ��aKJ/mol
C8H18(1)+25/2O2(g)=8CO2(g)+9H2O(1) ��H2= ��bKJ/mol
��д��25����101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)���Ȼ�ѧ����ʽ_________________________________��
(2)����CO2��H2Ϊԭ�ϣ��ں��ʵĴ���(��Cu/ZnO����)�����£�Ҳ�ɺϳ�CH3OH���漰�ķ�Ӧ�У�
�ף�CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H= �� 53.7kJ��mol-1 ƽ�ⳣ��K1
CH3OH(g)+H2O(g) ��H= �� 53.7kJ��mol-1 ƽ�ⳣ��K1
�ң�CO2(g)+H2(g) ![]() CO(g)+H2O(g) ��H= + 41.2kJ��mol-1 ƽ�ⳣ��K2
CO(g)+H2O(g) ��H= + 41.2kJ��mol-1 ƽ�ⳣ��K2
��CO(g)+2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��K=______(�ú�K1��K2�ı���ʽ��ʾ)���÷�Ӧ��H_____0(��������������С����)��
CH3OH(g)��ƽ�ⳣ��K=______(�ú�K1��K2�ı���ʽ��ʾ)���÷�Ӧ��H_____0(��������������С����)��
�����CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��___________(��д����)��
�۴����ͷ�Ӧ��ϵ�Ĺ�ϵ��������Կ�Ĺ�ϵһ�������и߶ȵ�ѡ���ԡ���������ʵ�飬����CO2��H2��ʼͶ�ϱȾ�Ϊ1��2.2��������ͬ��Ӧʱ��(t1min)��
�¶�(K) | ���� | CO2ת����(%) | �״�ѡ����(%) | �ۺ�ѡ�� |
543 | Cu/ZnO���װ����� | 12.3 | 42.3 | A |
543 | Cu/ZnO����Ƭ���� | 11.9 | 72.7 | B |
553 | Cu/ZnO���װ����� | 15.3 | 39.1 | C |
553 | Cu/ZnO����Ƭ���� | 12.0 | 70.6 | D |
�ɱ����е����ݿ�֪����ͬ�¶��²�ͬ�Ĵ�����CO2��ת��ΪCH3OH��ѡ����������Ӱ�죬�����ϱ��������ݽ�Ϸ�Ӧԭ������������ѡ��Ϊ___________(����ĸ����)��
(3)��CO��H2Ϊԭ�Ϻϳ��״��ķ�ӦΪ��CO(g)+2H2(g)![]() CH3OH(g)���������Ϊ2L�����������ܱ����������������У��ֱ���1molCO��2molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣��ͼΪ���������еķ�Ӧ�����е�5minʱH2���������ʾ��ͼ��������һ��������Ӧһ���ﵽƽ��״̬��
CH3OH(g)���������Ϊ2L�����������ܱ����������������У��ֱ���1molCO��2molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣��ͼΪ���������еķ�Ӧ�����е�5minʱH2���������ʾ��ͼ��������һ��������Ӧһ���ﵽƽ��״̬��
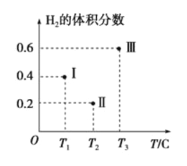
��0��5minʱ��������������CH3OH��ʾ�Ļ�ѧ��Ӧ����Ϊ_________________��
������������һ���ﵽƽ��״̬��������________(��д��������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�к������ʾ��ȫȼ��ʱ���ÿ�ȼ����X(X=A��B��C)�����ʵ���n(x)���������ʾ����O2�����ʵ���n(O2)��A��B�����ֿ�ȼ���壬C��A��B�Ļ�����壬��C��n(A)��n(B)Ϊ �� ��

A. 2��1 B. 1��2 C. 1��1 D. �����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ݱ������������أ�KH2PO4��������Ӧ�����ҹ����Ƶľ��ͼ����������������С����÷���ʯ����ѧʽΪCa5P3FO12)�Ʊ��������صĹ�����������ͼ��ʾ���������̲�����ʡ�ԣ���

��֪��ȡ����Ҫ��Ӧԭ����KCl+H3PO4![]() KH2PO4+HCl�����У���Ӧ������HCl�������л���ȡ����
KH2PO4+HCl�����У���Ӧ������HCl�������л���ȡ����
��ش��������⣺
��1�������н�����ʯ�����Ŀ����__________________________________��
��2������ʹ�ö��������մɲ��ʵķ��ڲ۵���Ҫԭ����___________________���û�ѧ����ʽ��ʾ����
��3������ƷN�Ļ�ѧʽ��____________���ڵõ�KH2PO4�����һϵ�в�����������Ҫ����______________________________�����ˡ�ϴ�ӡ��������
��4������1000kg��������Ϊ50.4%�ķ���ʯ����ѧʽΪCa5P3FO12)����ȡ�������ؾ��壬�����Ϊ80%���������Ͽ�����KH2PO4������Ϊ_______kg��
��5����ⷨ�Ʊ�KH2PO4��װ����ͼ��ʾ���õ��װ���У�a ������_______�����������������������������������ĵ缫��Ӧʽ��______________________________________��

��6����ҵ�ϻ������÷���ʯ�뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1500�����ɰ��ף�ͬʱ�ݳ�SiF4��CO,�÷�Ӧ�Ļ�ѧ����ʽΪ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪ������ij�������������������Ļ�ѧ��Ӧʾ��ͼ��

��1��ͼ�б�����ĸ�������У�_____����ø������ø�Ļ�ѧ������___________��������ɵ�λ��__________��
��2�����B�������ǣ���C��D������______________��________________��
��3�����й�����������������Ƚϵ�˵���д������___________________��
A�����ǵķ���ʽ��ͬ������ѧԪ�������ͬ
B��������ˮ�⣬������ȴ����
C��������ͬ���칹��
D���������ǵ��ǡ������Ƕ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����жԻ�ѧ��ѧ��Ӧ����������ȷ���ǣ�������
A.��ѧ��ѧ�������ƶ����Ͽ�ѧ�ķ�չ
B.��ѧ��ѧ��Ϊ��������Ľ���ṩ�����ı���
C.��ѧ��ѧ���˽⼲���IJ�����������Ϊ��
D.��ѧ��ѧʹ�����ܹ����õ�������Դ����Դ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��̱�����뻹ԭ������ʵ�ֵ��ǣ� ��
A.Cl2��Cl-B.Cl-��Cl2C.Cu2����CuD.CO32-��CO2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com