
����Ŀ���ݱ������������أ�KH2PO4��������Ӧ�����ҹ����Ƶľ��ͼ����������������С����÷���ʯ����ѧʽΪCa5P3FO12)�Ʊ��������صĹ�����������ͼ��ʾ���������̲�����ʡ�ԣ���

��֪��ȡ����Ҫ��Ӧԭ����KCl+H3PO4![]() KH2PO4+HCl�����У���Ӧ������HCl�������л���ȡ����
KH2PO4+HCl�����У���Ӧ������HCl�������л���ȡ����
��ش��������⣺
��1�������н�����ʯ�����Ŀ����__________________________________��
��2������ʹ�ö��������մɲ��ʵķ��ڲ۵���Ҫԭ����___________________���û�ѧ����ʽ��ʾ����
��3������ƷN�Ļ�ѧʽ��____________���ڵõ�KH2PO4�����һϵ�в�����������Ҫ����______________________________�����ˡ�ϴ�ӡ��������
��4������1000kg��������Ϊ50.4%�ķ���ʯ����ѧʽΪCa5P3FO12)����ȡ�������ؾ��壬�����Ϊ80%���������Ͽ�����KH2PO4������Ϊ_______kg��
��5����ⷨ�Ʊ�KH2PO4��װ����ͼ��ʾ���õ��װ���У�a ������_______�����������������������������������ĵ缫��Ӧʽ��______________________________________��

��6����ҵ�ϻ������÷���ʯ�뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1500�����ɰ��ף�ͬʱ�ݳ�SiF4��CO,�÷�Ӧ�Ļ�ѧ����ʽΪ________________________________________��
���𰸡� �������ʯ��ϡ���ᷴӦ�ĽӴ����,�ӿ컯ѧ��Ӧ���� 4HF+SiO2�TSiF4��+2H2O NH4Cl ����Ũ������ȴ�ᾧ 326.4 kg ���� 2H����2e����H2�� 4Ca5P3FO12 +21SiO2+30C![]() 20CaSiO3+3P4+SiF4��+30CO��
20CaSiO3+3P4+SiF4��+30CO��
������������ʯ(��ѧʽΪCa5P3FO12)��������Ũ���ᣬ��Ӧ��������ᡢ����ơ�����ȣ������Ȼ��غ����л���ȡ����KCl+H3PO4![]() KH2PO4+HCl����Ӧ������HCl�������л���ȡ�����л����к����Ȼ��⣬���백ˮ��Ӧ�����Ȼ�泥���˸���Ʒ��ҪΪ�Ȼ�泥�ˮ���к���KH2PO4������һϵ�в����õ�KH2PO4���塣
KH2PO4+HCl����Ӧ������HCl�������л���ȡ�����л����к����Ȼ��⣬���백ˮ��Ӧ�����Ȼ�泥���˸���Ʒ��ҪΪ�Ȼ�泥�ˮ���к���KH2PO4������һϵ�в����õ�KH2PO4���塣
(1)�����н�����ʯ���飬�����������ʯ��ϡ���ᷴӦ�ĽӴ�������ӿ컯ѧ��Ӧ���ʣ��ʴ�Ϊ���������ʯ��ϡ���ᷴӦ�ĽӴ�������ӿ컯ѧ��Ӧ���ʣ�
(2)��������ͼ����Ӧ������������ᣬ������ܹ���������跴Ӧ����˲���ʹ�ö��������մɲ��ʵķ��ڲۣ��ʴ�Ϊ��4HF+SiO2�TSiF4��+2H2O��
(3)������������������ƷN�Ļ�ѧʽΪNH4Cl���ڵõ�KH2PO4�����һϵ�в�����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ��ʴ�Ϊ��NH4Cl������Ũ������ȴ�ᾧ��
(4)1000kg��������Ϊ50.4%�ķ���ʯ(��ѧʽΪCa5P3FO12)�к���Ca5P3FO12������Ϊ504kg������PԪ���غ㣬�����Ͽ�����KH2PO4������Ϊ504kg��80%��![]() ��
��![]() =326.4 kg���ʴ�Ϊ��326.4 kg��
=326.4 kg���ʴ�Ϊ��326.4 kg��
(5)����ͼʾ��Ӧ����a������KH2PO4�����������b������a������a �������������������������ӷŵ������������缫��ӦʽΪ2H����2e����H2�����ʴ�Ϊ��������2H����2e����H2����
(6)�÷���ʯ�뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1500�����ɰ��ף�ͬʱ�ݳ�SiF4��CO����Ӧ�Ļ�ѧ����ʽΪ4Ca5P3FO12 +21SiO2+30C![]() 20CaSiO3+3P4+SiF4��+30CO�����ʴ�Ϊ��4Ca5P3FO12 +21SiO2+30C
20CaSiO3+3P4+SiF4��+30CO�����ʴ�Ϊ��4Ca5P3FO12 +21SiO2+30C![]() 20CaSiO3+3P4+SiF4��+30CO����
20CaSiO3+3P4+SiF4��+30CO����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�����Ӧ�õ������У���ȷ����
A.Si����������ά
B.���Dz����õķǽ���Ԫ�أ�����Ȼ���п���������̬����ʽ����
C.����Ʒ�ڿ������к�ǿ�Ŀ���ʴ������Ϊ���Ļ�ѧ���ʲ�����
D.�����£����ۡ����ۿɴ�����Ũ���ᡢŨ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.�л���X��һ����Ҫ���л��ϳ��м��壬�����������ϡ�Ϳ�Ϻ��ϼ��ȸ߾��Ϊ�о�X�������ṹ������������ʵ�飺
(1)�л���X������ͼΪ�� | |
| �л���X����Է���������________�� |
(2)��10.0 g X������O2�г��ȼ�գ���ʹ���������ͨ����������ˮCaCl2��KOHŨ��Һ��������ˮCaCl2����7.2 g��KOHŨ��Һ����22.0 g�� | �л���X�ķ���ʽ��______�� |
(3)��������ײⶨ���л���X�к���ȩ�����л���X�ĺ˴Ź�������ͼ����2�����շ壬�����֮����3��1�� | �л���X�Ľṹ��ʽ��__________�� |
II.д����![]() ��Ϊͬ���칹�壬��һ�����ֻ�����ֵķ������Ľṹ��ʽ�����ƣ�________________��___________��
��Ϊͬ���칹�壬��һ�����ֻ�����ֵķ������Ľṹ��ʽ�����ƣ�________________��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ����Һ�����ܺ���Al3+��Fe3+��K+��NH4+��Mg2+��Cu2+�������е�һ�ֻ��֡��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ��ͼ����ʾ��
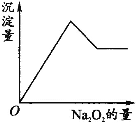
���ƶϣ�
��1��ԭ��Һ��һ������________��
��2��һ��������________��
��3�����ܺ���________��
��4��Ϊ�˽�һ��ȷ�����ܺ��е����ӣ�Ӧ���ӵ�ʵ�鷽��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����н�����ұ�������������(����)
A.����ͨ��Al2O3������B.����HgO
C.������ڵ�MgCl2D.���ۺ�Fe2O3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ��F���п���������Ѫ�ǡ���Ѫѹ�ȶ���������ԣ���ϳ�·�����£�

��֪��M�Ľṹ��ʽΪ��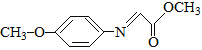 ��
��
��ش��������⣺
��1��A�Ļ�ѧ������_______________��B�Ļ�ѧʽ��________________��
��2��C�й����ŵ�������__________________________��
��3��д��F�Ľṹ��ʽ__________________________��
��4����֪A��һ�������������ɿɽ���ľ�������д���÷�Ӧ��ѧ����ʽ��______________________________��
��5����������������M��ͬ���칹����_______��(���������칹)��
�� �ܹ�����������Ӧ��
�� ��������(�CNO2)��������ֱ�����ڱ����ϡ�
�� ���б����ұ�����ֻ������ȡ������
���к˴Ź�������Ϊ������ҷ����֮��Ϊ6��2��2��1�Ľṹ��ʽΪ________��д��һ�ּ��ɣ���
��6��д������ȩΪԭ���Ʊ��߷��ӻ�����۱�ϩ��ĺϳ�·�ߣ����Լ���ѡ����______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ijЩ���ӵļ��鼰����һ����ȷ����
A. ����ϡ���������ɫ���壬������ͨ�����ʯ��ˮ�У���Һ����ǣ�һ����CO![]()
B. �����Ȼ�����Һ�а�ɫ�����������ټ����ᣬ��������ʧ��һ����SO![]()
C. ��������������Һ�����ȣ�������������ʹʪ��ĺ�ɫʯ����ֽ������һ����NH![]()
D. ����̼������Һ������ɫ�������ټ������ɫ������ʧ��һ����Ba2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2FeO4�����������ص���Ҫԭ�ϣ�ͬʱҲ��һ�����͵ĸ�Ч��ˮ�����ڹ�ҵ��ͨ��������ͼװ������Na2FeO4�������й�˵������ȷ����

A. �Ҳ�缫��Ӧ����ʽ��Fe+8OH--6e-=FeO42-+4H2O
B. ���Ϊ�����ӽ���Ĥ����Cu�缫����1mol����ʱ����2molNa+ͨ�������ӽ���Ĥ
C. ���Խ�����������������Ʋ��䵽��װ���в����Ա�֤װ����������
D. Na2FeO4����ǿ�������Ҳ���ΪFe3+����˿�������Na2FeO4��ȥˮ�е�ϸ������������Լ�Ca2+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йس�������Ʒ��ʹ���У�����Ϊ�������ǣ�������
A.ʢ��ʳ��
B.����ˮ
C.�ý���˿��ϴ������۹�
D.�ü�ˮϴ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com