
���ͷ���һ�ֻ�ѧ���ɼ�������С�մ��ۣ�̼����泥�������[KAl(SO4)2?12H2O]�е�����������ɡ�ijС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣���������ʵ�顣
��������衿
��1������1����С�մ�ͳ������
����2����С�մ���������
����3����__________________________���
�����������̡�
Ϊ̽����Ʒ�Ƶķ��ͷ۵ijɷ֣�ijͬѧ�������ʵ�飬�õ���������
��2����ϲ���١��۷���������AΪ________���÷��ͷ۵ijɷ�Ϊ__________________��
��3��������ٺ͢ڲ������䣨����Ҳ��ͬ�����������������ϡ�����Ϊ�����Ȼ�����Һ���۲쵽�а�ɫ�������ɣ��ܷ�ȷ�����ͷ۵ijɷֲ�˵�����ɣ�________________�� ____________________________________________________________________��
��4����Ʒ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2����������ʵ����֤��
ʵ����������Ʒ��ѡ����ѡ�Լ���ϡ���ᡢ0.1 mol/LNaOH��Һ��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ������Ʒ��������������� ����Һ�ֳ����ݣ��ֱ�װ��A��B�Թ��С� | |
| ����2��_____________________________ __________________________________ | ________________________֤����Na+���� �ͷ�����NaHCO3�� |
| ����3��_____________________________ ___________________________________ | ___________________________________ _______����ϲ���2�еĽ��ۣ�����2������ |
��16�֣���1�����ۺ�������2�֣�
��2����������NH3����2�֣� NaHCO3��NH4HCO3��2�֣�д����Ҳ���֣�
��3�����ܣ�1�֣�����Ϊ����NaHCO3�ֽ��Na2CO3��Na2CO3������������BaC12��Һ��Ӧ���ɰ�ɫ��������2�֣� ע����˼�ӽ�Ҳ�÷�
��4����7�֣�����2���ýྻ�IJ�˿պȡA�е���Һ����
�ƾ������������գ��۲���ɫ��2�֣���ɫ�ʻ�ɫ��1�֣� ����3��������1����B�Թ�����εμ�
0.1 mol/L NaOH��Һ��2�֣������а�ɫ�������ɣ����Ȳ�����ɫ����������ܽ⣩��1�֣���֤�����ͷ�������������1�֣�
������2����պȡA�е���Һ����պȡB��Һ�����ھƾ������������գ�1�֣�������ɫ�ܲ����۲죨1�֣� ��ɫ����ɫ��1�֣���֤�����ͷ��к���������1�֣�
ע������2�У����ýྻ�IJ�˿պȡ����Һ����1�֣����ھƾ��ƻ��������ա���1�֡�
ֻд��պȡ����Һ���ø��ֵ㲻�÷֣����ھƾ��ơ��ƾ���ƻ��������ա������֡�
����3�У�������ɫ��Ӧ����������ϴ�Ӳ�˿������NaOH��Һ���飬��0.lmol/L NaOH
��Һ����1�֣�����εμӡ���1�֡�
���������������1�����������֪����ͬƷ�Ƶķ��ͷۿ���С�մ�ͳ�����ɣ�Ҳ������С�մ��������ɣ��������ɳ��ۺ�������ɣ�������֪�ļ���1������2�ƶϼ���3Ϊ���ۺ���������2������NaHCO3��NH4HCO3��Al2(SO4)2?12H2O����Ҫ���ʿ�֪��NH4HCO3����Σ�����ʯ�һ����ĥ���Էų���������������������ʯ����ĥ�����ܷų����壬��AΪNH3�����ͷۼ�һ�����г��ۣ�NH4HCO3�����ּ��Ⱥ���ȫ��Ϊ�����ݳ���NaHCO3���������ȱ�ΪNa2CO3�����CO2��H2O��Na2CO3������������Ӧ���ɶ�����̼���塢NaCl��H2O����Al2(SO4)2?12H2O����ˮ�����Al(OH)3�������϶�������������ɴ��ƶ�BΪCO2���÷��ͷ�һ������С�մ����Լ�Ʒ�Ƶķ��ͷ۵���Ҫ�ɷ���NaHCO3��NH4HCO3����3��Ba(OH)2�����������η�Ӧ��������ɫ�����ᱵ������Ҳ����̼���η�Ӧ�����ɰ�ɫ��̼�ᱵ��������˲���ȷ�����ͷ��к�����������С�մ�4������ʵ�鷽���в���2�Ľ������ƿ�֪������2���ýྻ�IJ�˿պȡA�е���Һ���پƾ������������գ��������ɫ�ʻ�ɫ��֤����Na+�����ͷ�����NaHCO3�����ڼ���2�Ƿ��ͷ���С�մ��������ɣ�����3�Ľ�����֤�����ͷ���Al2(SO4)2?12H2O�����������ʼ��ṩ�Լ��������ƶϣ�����3�����ʵ�鷽��֤�����ͷ��к���Al3+�����Ӧ��B�Թ�����εμ�0.1mol/LNaOH��Һ���۲쵽��ɫ�������Ȳ�����ɫ����������ܽ⣬֤����Al3+�����ͷ�����������
���㣺����̽��ʵ�鷽������Ƶ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1MgSO4��Һ��0.5mol��L-1NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL�Ŀ���ƿ�У��������������¶ȿ�����50�档
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40�����ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1~5����
�ٲ���2�����¶���50�棬�Ϻõļ��ȷ����� ��
�ڲ���3����MgCO3��nH2O���������ӷ���ʽΪ ��
�۲���4�����Ƿ�ϴ�Ӹɾ��ķ����� ��
��2���ⶨ�ϳɵ�MgCO3��nH2O�е�nֵ��
����1.000g̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м���ˮ����ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4~5h����Ӧ���ڽ��¶�����30�棬�����ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�
��ͼ������������� ��
��������Ӧ����Ҫ���µ�30�棬��ҪĿ���� ��
����3��ʵ����ÿ1.000g̼��þ���������CO2ƽ��ֵΪa mol����nֵΪ ���ú�a�ı���ʽ��ʾ����
��3����ȡ100g���������Ʒ�������ط���������������ͼ��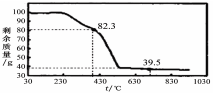
��������ºϳɵľ����У�n= ��ѡ�1��2��3��4��5����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧС���ͬѧ������̼��Ϊ�缫����Ȼ�ͭ��Һʱ��������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ����������¹��̣�
�������
ͭ�Ļ�������ɫ�������£�
| ���� | ��ɫ������ | ���� | ��ɫ������ |
| ������ͭCu(OH)2 | ��ɫ���岻����ˮ | ����ͭ��CuSO4�� | ��Һ����ɫ |
| ������ͭ��CuO�� | ��ɫ���岻����ˮ | �Ȼ�ͭ��CuCl2�� | ��Һ����ɫ��ϡ��Һ����ɫ |
| �Ȼ���ͭ��CuCl�� | ��ɫ���岻����ˮ | ��ʽ�Ȼ�ͭ | ��ɫ���岻����ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ư����һ�ֳ��õ���������
��1����ҵ������Ư�۷�Ӧ�Ļ�ѧ����ʽΪ��________________ __��Ư�۵���Ч�ɷ�Ϊ ��
��2��ij̽��С����г��Ϲ�����һ����װ�����Ư�ۣ��Ը�Ư�۵ijɷֽ���̽�������������Լ������ʵ�鷽��������ʵ�顣���ڴ�������ʵ�鱨�档
��ѡ�Լ���2mol��L��1NaOH��Һ��2mol��L��1HCl��Һ��2mol��L��1HNO3��Һ��0.5mol��L��1BaCl2��Һ��0.01mol��L��1AgNO3��Һ������ʯ��ˮ��ʯ����Һ����̪��Һ������ˮ��
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����Ư��������������ˮ����ֽ��裬���ã����ˣ��ó�������Һ�� | |
| ����2���������������2mol��L��1HCl��Һ��������������ͨ�� | ���� ���ۣ� |
| ����3��ȡ��Һ��װA��B��֧�Թܡ���A�Թܣ� | ������Һ�ȱ��ɫ��Ȼ����ɫ�� ���ۣ� |
| ����4����B�Թܣ� | ��������ɫ������ ���ۣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg(ClO3)2��6H2O���������£�
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
�����ֻ�������ܽ��(S)���¶�(T)�仯������ͼ��ʾ��
��1����������Ҫ����Ҫ���������� ����MgO�����������������Ҫ�ɷ�Ϊ ��
��2������BaCl2��Ŀ���dz�ȥSO42������μ���SO42���ѳ�����ȫ���� ��
��3������NaClO3������Һ������ӦΪ��MgCl2��2NaClO3��Mg(ClO3)2��2NaCl���ٽ�һ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ���� ���� ���� ���ܹ��ˡ�ϴ�ӡ�
��4����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10 mL����ƿ�У�����10 mLϡ�����20 mL 1.000 mol��L��1��
FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol��L��1 K2Cr2O7 ��Һ�ζ����յ㣬�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7 ��Һ15.00 mL��
��д������2�з�����Ӧ�����ӷ���ʽ��
�ڲ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͭ��ʯ��ͭԪ�غ����ϵͣ��Һ�������þ���Ƶ��������ӡ�ijС����ʵ�������ý���-��ȡ���Ʊ�����ͭ��
��1������IΪ_______������II�õ��IJ����������ձ�_______
��2������II������III����ҪĿ����_______������ͭԪ�ء�
��3��С���Ա����CuSO4��Һ��Na2CO3��Һ��Ϸ�Ӧ���Ʊ�������ľ�ķ�����Cu2(OH)2CO3����Һ�����ʵ�鷢��������ɫ����Һ��ɫ���в��죬�������ϱ��������������������Ʋ�ͬʹ���л��н϶�Cu(OH)2��Cu4(OH)6SO4��
��֪Cu(OH)2��Cu2(OH)2CO3��Cu4((OH)6SO4��������ˮ����������ֽ��¶�����Ϊ 80�桢200�桢300�档
���ʵ���������Һ�ɷ֣���ɱ������ݡ�
��ѡ�Լ���2mol?L��1HCl��1 mol?L��1H2SO4��0.1 mol?L��1NaOH��0.1 mol?L��1 BaCl2������ˮ����������Ʒ��ѡ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ��������Һ�����ˣ����ϴ�Ӻ�ȡ�������Թ��У�_______________________________________________________________ | ˵������Һ�л�__________________________________________,��Cu4( OH)6SO4�� |
| ����2����ȡ��������Һ���Թ��У�____________________________ | ______________�� ˵������Һ�л���Cu( OH) 2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ��Դ�Ŀ����������ǵ�ǰ��ѧ�о���һ����Ҫ������ͼ��ʵ����ģ��þ����������ͼ��
������������ͼ���ش��������⣺
��1������I�������� ��
��2�������Тڷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3��������м���ʱͨ��HCl������������ ��
��4������ݵ����ӷ�Ӧ����ʽΪ ��
��5�������������ݵĸ������������������������ߺ�ˮ���ۺ������ʡ���AΪ���������е�ij��֣���A��B�ֱ�Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ʵ�����Ʊ����ռ������SO2�������������¡�װ�ã�����SO2���������������Ӹ�������˳��Ϊ��ӣ� ���ӣ� ���ӣ� ���ӣ� ���ӣ棨��ӿ���ĸ�� �� ��
| A��b c d e | B��d e b c | C��d e c b | D��e d b c |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com