
����Ŀ��(1)���з�Ӧ�����ڼ��˾���Ƿ�ƺ��ʻ��2![]() +3CH3CH2OH+16H++13H2O
+3CH3CH2OH+16H++13H2O![]() 4[Cr(H2O)6]3++3CH3COOH
4[Cr(H2O)6]3++3CH3COOH
�������[Cr(H2O)6]3+�У���Cr3+�γ���λ����ԭ����___(��Ԫ�ط���)��
��CH3COOH��Cԭ�ӹ���ӻ�����Ϊ___��1 mol CH3COOH���Ӻ�����������ĿΪ___��
(2)CS2�����У�Cԭ�ӵ��ӻ����������____��д��������CS2������ͬ�ռ乹�ͺͼ�����ʽ������____��
(3)ʯīϩ��һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ��
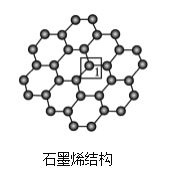
����ʯīϩ�У�1��C���ӻ���ʽ��____����C������C�γɵ�C-C����____(����>������<������=��)ʯīϩ����1��C����C�γɵ�C-C���ǡ�
���𰸡�O sp3��sp2 7 NA sp SCN- OCN-�� sp3 <
��������
(1)�����[Cr(H2O)6]3+��Cr3+Ϊ�������ӣ�H2OΪ���壻
(2)CH3COOH��Cԭ�ӷֱ��γ�4����3��������û�й¶Ե��ӣ�CH3COOH�����к���1��C-C��3��C-H��1��C-O��1��C=O��1��O-H�Ȼ�ѧ����
(3)CS2���ӵĽṹʽΪS=C=S����������¶Ե������жϼ۲���Ӷ������ж��ӻ����ͣ���CS2������ͬ�ռ乹�ͺͼ�����ʽ������Ϊ�ȵ����壬Ӧ����3��ԭ�ӣ��۵�����Ϊ16��
(4)����ʯīϩ��1��Cԭ���γ�4���������۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�Cԭ���ӻ���ʽ������ʯīϩ��1��̼ԭ�Ӻ�3��C��1��Oԭ���γ�������ṹ��ʯīϩ��ÿ��Cԭ�Ӻ�����3��̼ԭ���γ�ƽ��ṹ���ݴ˷����жϡ�
(1)�������[Cr(H2O)6]3+��Cr3+Ϊ�������ӣ�H2OΪ���壬Oԭ���ṩ�¶Ե��ӣ���Cr3+�γ���λ�����ʴ�Ϊ��O��
��CH3COOH��Cԭ�ӷֱ��γ�4����3����������û�йµ��Ӷԣ��ֱ�Ϊsp3�ӻ���sp2�ӻ���CH3COOH�����к���1��C-C��3��C-H��1��C-O��1��C=O��1��O-H��ѧ������1mol CH3COOH�����к�����������ĿΪ7 NA��7��6.02��1023���ʴ�Ϊ��sp3��sp2��7 NA��7��6.02��1023��
(2)CS2������Cԭ���γ�2���������¶Ե�����Ϊ![]() =0����CΪsp�ӻ���
=0����CΪsp�ӻ���
��CS2������ͬ�ռ乹�ͺͼ�����ʽ������Ϊ�ȵ����壬Ӧ����3��ԭ�ӣ��۵�����Ϊ16��������SCN-��OCN-�ȣ��ʴ�Ϊ��sp��SCN-��OCN-�ȣ�
(3)����ʯīϩ�У�1��C�γ�3��C-C��1��C-O����Cԭ����sp3�ӻ���Ϊ�����幹�ͣ���ʯīϩ�е���1��C�γ�C-C���ĵ�Cԭ����sp2�ӻ���Ϊƽ�������ι��ͣ�������ʯīϩ��1��C������C�γɵ�C-C���ǣ�ʯīϩ����1��C����C������C�γɵ�C-C���ǣ��ʴ�Ϊ��sp3������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Na2CO3��10H2O����,����0.2 mol��L-1��Na2CO3��Һ480 mL��
(1)Ӧ��ȡNa2CO3��10H2O���������:___________________��
(2)�������в�����������Һ��Ũ�Ȳ�����Ӱ��,�����������:
��Na2CO3��10H2O����ʧȥ�˲��ֽᾧˮ
�����������������ij���������������(ʹ������)
��̼���ƾ��岻��,���л����Ȼ���
�ܳ���̼���ƾ���ʱ������������
������ƿδ������ʹ��
��������������ҺŨ��ƫ�ߵ���______________(�����,��ͬ),ƫ�͵���_____________,��Ӱ�����________________��
(3)���в�����,����ƿ�����߱��Ĺ�����__________(�����)��
A������һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E����ȡһ�������Һ��
F�����������ܽ��������
(4)ijͬѧ���ù���Na2CO3��������Na2CO3��Һ�Ĺ�����ͼ��ʾ:


����Ϊ��ͬѧ�Ĵ�������________(�����)��
A��1�� B��2�� C��3�� D��4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ����٤��������ֵ������˵����ȷ����
A.54 g Al������ϡ���ᷴӦ��ת�Ƶĵ�����Ϊ6 NA
B.��״���£�1.8 g H2O��ռ�����ԼΪ2.24 L
C.���ʵ���Ũ��Ϊ0.5 mol��L��1��MgCl2��Һ�У�����Cl������ΪNA
D.1 mol����������������Ӧ��ʧȥ�ĵ�����Ϊ2 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ϸ���ڲ����л�������ĸ���ͼ����ش��������⡣

��1��С�������еĴ�������c��________���˺Ͷ���ϸ���еĴ�������c��________��
��2�����b�Ķ������ɰ�����ͨ����ˮ�������Ӷ��ɣ���ˮ������ָ____________________��
��3���������ƬҶ______����ڡ����⡱����ϸ��������ͨ��Ⱦɫ���۲�DNA��e�ķֲ���ʵ�����������Ҫ������ˮ�⣬Ŀ���иı�ϸ��Ĥ��ͨ�ԣ�����Ⱦɫ������ϸ����ͬʱʹ______________________������DNA��Ⱦɫ����ϡ�
��4�����������d��_______����ѪҺ��֬�ʵ����䣬ά����D����Ч�شٽ�__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����500 mL NH4HCO3��Na2CO3�Ļ����Һ�ֳ���ȷݣ�ȡһ�ݼ�������NaOH��Һ����ַ�Ӧ������ a mol NaOH ����ȡһ�ݼ����������ᣬ��ַ�Ӧ������b mol HCl����û����Һ��c(Na��)Ϊ (����) ����֪��NaHCO3 + NaOH = Na2CO3 + H2O��
A.��![]() ��mol/LB.��
��mol/LB.��![]() ��mol/L
��mol/L
C.��10b-5a�� mol/LD.(2b-a) mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Ʒ��Ҫ��KCl������MgCl2��CaCl2��MgSO4�ȣ���Ҫ�����ᴿ�õ�KCl��
��1��ʵ���Ҳ��ó������ⶨ��Ʒ��MgSO4�����������������������£�
��ȡ10g��Ʒ����ˮ�У�����������BaCl2��Һ����ֽ��跴Ӧ���ڽ����û������ˡ�ϴ�ӡ��۽�������ͬ��ֽȡ���������������������أ���ֽ��ȫת����CO2��ˮ���������ֽ⣩����ȴ��������ó�������Ϊ0.466g��
�жϳ����Ƿ�ϴ�Ӹɾ��ķ�����_______________����Ʒ��MgSO4����������Ϊ________��
��2�����м����Լ����������K2CO3����Na2CO3����NaOH����KOH����Ba(OH)2����BaCl2��Ϊ��Ч��ȥ��Ʒ�е����ʣ�������Լ���˳���������____________��
A���ߢۢ� B���ߢݢ� C���ڢ� D���ݢڢ�
���˳�ȥ��������Ҫ������Լ�Ϊ_________ (�����)��Ȼ����������ᾧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ֱ�ȼ�յ����ʵ��������и������ʣ�������������������ȵ��ǣ� ��
��C2H2��C2H4O ��C4H8��C6H12O6 ��C7H8��C6H12 ��HCOOCH3��CH3COOH��
A���٢ۢ� B���٢ڢۢ� C���٢� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����ա�
I�����и����е������л�������ǣ�(A)��ͬ�����ʣ�(B)ͬϵ�(C)ͬ���칹�塣���ж�����֮��Ĺ�ϵ(��A��B��C���)
�� 2��������Ͷ���________________�� ���������2��2����������____________���۶Զ��ױ���1,4�����ױ�____________ �� �� 1����ϩ�ͻ�����________________��
II���������ŵIJ�ͬ�����Զ��л�����з��࣬������Ҫ��Ĵ����ں����ϡ�
�� CH3CH2CH2COOH �� ![]() ��
��![]()
�� ![]() ��
�� ![]() ��
�� 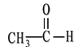
(1)��������__________��
(2)±������____________��
(3)�ӣ�_______________��
(4)ȩ��______________��
(5)���______________��
(6)����_______________��
III��(1)�����ǻ�����ϩ�ĵ���ʽ���ǻ� __________����ϩ __________��
(2)��ϵͳ�������������л�������
![]() ___________________________��
___________________________��
��![]() ___________________________________��
___________________________________��
(3)��Է�������Ϊ114����һ�ȴ���ֻ��һ�ֵ������Ľṹ��ʽ ___________�����ʵ�����Ϊ________________��
(4)ij��1���Ӻ���50�����ӣ�����ֻ����һ�ֽṹ��Ȳ������õ���������ļ���ʽΪ _____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ��̽��Cl2��NO2��NaOH��Һ�еķ�Ӧ����ͨ���ʵ�������Cl2��NO2����������ӦCl2+2NO2+4NaOH=2NaNO3+2NaCl+2H2O��
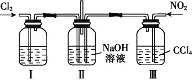
����������ȷ����
A. ʵ�������ö���������3 mol��L-1�����Ṳ���Ʊ�����
B. װ�â���ʢ�ŵ��Լ���Ũ���ᣬ�����Ǹ�������
C. װ�â�������DZ��ڿ���ͨ��NO2����
D. ���Ʊ���NO2�к���NO��Ӧ���������ͨ��ˮ���Գ�ȥNO
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com