
ЮЊСЫЬсИпзЪдДРћгУТЪЃЌМѕЩйЛЗОГЮлШОЃЌЛЏЙЄМЏЭХНЋюбГЇЁЂТШМюГЇКЭМзДМГЇзщГЩВњвЕСДЃЌШчЭМЫљЪОЁЃ
ЧыЬюаДЯТСаПеАзЁЃ
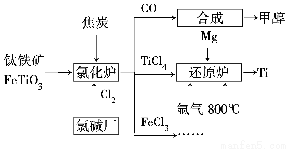
(1)юбЬњПѓНјШыТШЛЏТЏЧАЭЈГЃВЩШЁЯДЕгЁЂЗлЫщЁЂКцИЩЁЂдЄШШЕШЮяРэЗНЗЈДІРэЃЌЧыДгдРэЩЯНтЪЭЗлЫщЕФзїгУЃК_______________________________________
вбжЊТШЛЏТЏжаТШЦјКЭНЙЬПЕФРэТлгУСЯЮяжЪЕФСПжЎБШЮЊ7ЁУ6ЃЌдђТШЛЏТЏжаЛЙдМСЕФЛЏбЇЪНЪЧ___________________________ЁЃ
(2)вбжЊЃКЂйMg(s)ЃЋCl2(g)=MgCl2(s)ІЄHЃНЃ641 kJ/mol
Ђк2Mg(s)ЃЋTiCl4(s)= 2MgCl(s)ЃЋTi(s)ІЄHЃНЃ512 kJ/mol
дђTi(s)ЃЋ2Cl2(g)=TiCl4(s)ЁЁІЄHЃН________ЁЃ
(3)ыВЦјЭЈШыЛЙдТЏжаВЂВЛВЮгыЗДгІЃЌЭЈШыыВЦјЕФзїгУЪЧ___________________________
(4)вдМзДМЁЂПеЦјЁЂЧтбѕЛЏМиШмвКЮЊдСЯЃЌЪЏФЋЮЊЕчМЋПЩЙЙГЩШМСЯЕчГиЁЃвбжЊИУШМСЯЕчГиЕФзмЗДгІЪНЮЊ2CH3OHЃЋ3O2ЃЋ4OHЃ=2CO32ЁЊЃЋ6H2OЃЌИУЕчГижае§МЋЩЯЕФЕчМЋЗДгІЪНЮЊ_________________________________________ЁЃ
ЙЄзївЛЖЮЪБМфКѓЃЌВтЕУШмвКЕФpH________(ЬюЁАМѕаЁЁБЁЂЁАдіДѓЁБЛђЁАВЛБфЁБ)ЁЃ
(1)діДѓЗДгІЮяМфЕФНгДЅУцЛ§ЃЌЬсИпЗДгІЫйТЪЁЁCЁЂFeTiO3
(2)Ѓ770 kJ/mol
(3)MgКЭTiЖМгаЧПЛЙдадЃЌдкыВЦјЗеЮЇжаПЩЗРжЙMgЁЂTiБЛбѕЛЏ
(4)O2ЃЋ2H2OЃЋ4eЃ=4OHЃ(Лђ3O2ЃЋ6H2OЃЋ12eЃ=12OHЃ)ЁЁМѕаЁ
ЁОНтЮіЁП(1)ЗлЫщЗДгІЮяЃЌПЩвддіДѓЦфБэУцЛ§ЃЌДгЖјдіДѓЗДгІЮяжЎМфЕФНгДЅУцЛ§ЃЌЬсИпЗДгІЫйТЪЃЛТШЛЏТЏжаЗЂЩњЕФЗДгІЮЊ6CЃЋ7Cl2ЃЋ2FeTiO3=6COЃЋ2TiCl4ЃЋ2FeCl3ЃЌгЩДЫПЩжЊCКЭFeTiO3ЮЊЛЙдМСЁЃ(2)ИљОнИЧЫЙЖЈТЩЃЌгЩЂйЁС2ЃЂкЕУЃКTi(s)ЃЋ2Cl2(g)=TiCl4(s)ЃЛІЄHЃНЃ770 kJ/molЁЃ(3)ыВЦјаджЪВЛЛюЦУПЩвдЗРжЙMgКЭTiЕШОпгаЧПЛЙдадЕФЮяжЪБЛбѕЛЏЁЃ(4)ИљОнИУЕчГиЕФзмЗДгІЪНПЩжЊЃЌЗДгІЙ§ГЬжаВЛЖЯЯћКФOHЃЃЌдђИУЕчГиЙЄзївЛЖЮЪБМфКѓЃЌШмвКЕФpHНЋМѕаЁЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжИДЯА зЈЬт1ЮяжЪЕФзщГЩЗжРраджЪМАЛЏбЇгУгяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЙигкЫсЁЂМюЁЂбЮЁЂбѕЛЏЮяЕФдЊЫизщГЩЕФЫЕЗЈжае§ШЗЕФЪЧ (ЁЁЁЁ)ЁЃ
AЃЎЫсЁЂМювЛЖЈКЌгаЧтдЊЫиЃЌбЮвЛЖЈВЛКЌЧтдЊЫи
BЃЎМюЁЂбЮПЩФмКЌбѕдЊЫиЃЌЫсвЛЖЈКЌбѕдЊЫи
CЃЎЫсЁЂМюЁЂбЮПЩФмЖМВЛКЌН№ЪєдЊЫи
DЃЎЫсадбѕЛЏЮявЛЖЈЪЧгЩЗЧН№ЪєдЊЫиКЭбѕдЊЫизщГЩ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжИДЯА зЈЬт10ГЃМћЗЧН№ЪєдЊЫиМАЦфживЊЛЏКЯЮяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЪЕбщЬт
SiO2ЁЂSO2КЭCO2ЖМЪЧЫсадбѕЛЏЮяЃЌЫќУЧЕФЛЏбЇаджЪОпгавЛЖЈЕФЯрЫЦадЃЛMgКЭNaЕФЛЏбЇаджЪвВОпгавЛЖЈЯрЫЦадЁЃ
ФГаЫШЄаЁзщгУШчЭМЫљЪОзАжУНјааMgгыSO2ЗДгІЕФЪЕбщЁЃ

(1)бЁдёжЦШЁSO2ЕФКЯЪЪЪдМС________(ЬюБрКХ)ЁЃ
ЂйХЈHClЁЁЂкХЈH2SO4ЁЁЂлNa2SO3ЙЬЬхЁЁЂмCaSO3ЙЬЬх
(2)ЩЯЪізАжУЛЙПЩгХЛЏЃЌгХЛЏЕФЗНЗЈЪЧ________________________________________ЃЌзАжУCжаNaOHШмвКЕФзїгУЪЧ___________________________________________________________
(3)МзЭЌбЇЭЦВтMgгыSO2ЕФЗДгІКЭMgгыCO2ЕФЗДгІЯрЫЦЃЌдђИУЗДгІЗНГЬЪНЮЊ_________________________________________ЃЛ
ввЭЌбЇЕФЭЦВтЪЧЃК2MgЃЋ3SO2 2MgSO3ЃЋSЃЛБћЭЌбЇЕФЭЦВтЪЧЃК3MgЃЋSO2
2MgSO3ЃЋSЃЛБћЭЌбЇЕФЭЦВтЪЧЃК3MgЃЋSO2 2MgOЃЋMgSЃЌвЊбщжЄМзЁЂввЁЂБћШ§ЮЛЭЌбЇЕФЭЦВтЪЧЗёе§ШЗЃЌЖЁЭЌбЇзїШчЯТЪЕбщЬНОПЃК
2MgOЃЋMgSЃЌвЊбщжЄМзЁЂввЁЂБћШ§ЮЛЭЌбЇЕФЭЦВтЪЧЗёе§ШЗЃЌЖЁЭЌбЇзїШчЯТЪЕбщЬНОПЃК
вбжЊЃКMgSO3КЭMgSЖМЮЂШмгкЫЎЃЌФмгыбЮЫсЗЂЩњИДЗжНтЗДгІЗХГіЦјЬхЃЛH2SЦјЬхЭЈШыCuSO4ШмвКжаГіЯжКкЩЋГСЕэЁЃ
ЯобЁЪдМСЃК2 molЁЄLЃ1бЮЫсЁЂ2 molЁЄLЃ1ЯѕЫсЁЂеєСѓЫЎЁЂ2 molЁЄLЃ1 NaOHШмвКЁЂЦЗКьШмвКЁЂГЮЧхЪЏЛвЫЎЁЂ2 molЁЄLЃ1 CuSO4ШмвКЃЛвЧЦїКЭгУЦЗздбЁЁЃ
ађКХ | ЪЕбщВНжш | дЄЦкЯжЯѓКЭНсТл |
Ђй | ШЁЩйСПЗДгІКѓЫљЕУЙЬЬхгкЪдЙмжа |
|
Ђк | ЯђЪдЙмжаЕФЙЬЬхТ§Т§ЕЮМг____________ЃЌЪдЙмПкШћЩЯДјЕМЙмЕФЕЅПзШћЃЌВЂНЋЕМЙмЭЈШыЪЂга________ЕФЪдЙмжа | ШєЪдЙмжаЕФ________ЃЌдђБћЭЌбЇЭЦВте§ШЗЃЌШєЪдЙмжаЕФЙЬЬхЮДЭъШЋШмНтЃЌЧв________ЃЌдђввЭЌбЇЭЦВте§ШЗ |
ИљОнЩЯЪіЪЕбщЬНОПЃЌФмжЄУїМзЭЌбЇЭЦВте§ШЗЕФВйзїКЭдЄЦкЯжЯѓЪЧ
_____________________________________________________________ЁЃ
(4)ЩЯЪіЪЕбщашвЊ100 mL 2 molЁЄLЃ1ЕФбЮЫсЃЌХфжЦЪБбЁгУ________(бЁЬю10 mLЁЂ25 mLЁЂ50 mLЛђ100 mL)СПЭВСПШЁ36.5%УмЖШЮЊ1.19 gЁЄmLЃ1ЕФХЈбЮЫсЕФЬхЛ§ЮЊ________mLЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжГхДЬЗЧбЁдёЬтдіЗжзЈСЗ злКЯЪЕбщЩшМЦСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЪЕбщЬт
ФГЛЏбЇбЇЯАаЁзщЩшМЦЯТЭМЪЕбщзАжУ(МаГжзАжУТдШЅ)жЦБИCl2ЃЌВЂЬНОПТШЦјЕФЯрЙиаджЪЁЃ

(1)ШєAзАжУжаЙЬЬхвЉЦЗЮЊKClO3ЃЌдђЗДгІжаУПЩњГЩ1 mol Cl2ЪБзЊвЦЕчзгЕФЮяжЪЕФСПЮЊ________molЁЃ
(2)зАжУBМШФмГ§ШЅТШЦјжаЕФТШЛЏЧтЃЌЛЙФмМьВтЪЕбщЙ§ГЬжазАжУCЪЧЗёЗЂЩњЖТШћЁЃШєCжаЗЂЩњЖТШћЃЌдђBжаНЋЙлВьЕНЕФЯжЯѓЪЧ_______________________________________ЁЃ
(3)зАжУCЕФзїгУЪЧбщжЄТШЦјЪЧЗёОпгаЦЏАзадЃЌЂёДІЪЧЪЊШѓЕФгаЩЋВМЬѕЃЌдђЂђЁЂЂѓДІгІМгШыЕФЮяжЪЗжБ№ЪЧ________ЁЂ________ЁЃ
(4)ЩшМЦзАжУDЁЂEЕФФПЕФЪЧБШНЯТШЁЂфхЁЂЕтЕФЗЧН№ЪєадЁЃЧыМђЪіФмЫЕУїТШЁЂфхЁЂЕтЗЧН№ЪєадЧПШѕЕФЪЕбщВйзїМАЯжЯѓЃК_____________________________________________ЁЃ
(5)ЧыгУЛЏбЇЗНГЬЪНЫЕУїзАжУFЕФзїгУЃК_______________________________________ЁЃ
(6)МзЭЌбЇЬсГіЃЌзАжУFжаЕФЪдМСПЩИФгУзуСПЕФNa2SO3ШмвКЃЌввЭЌбЇШЯецЫМПМКѓШЯЮЊДЫЗЈВЛПЩааЁЃЧыгУРызгЗНГЬЪННтЪЭввШЯЮЊВЛПЩааЕФдвђЃК_______________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжГхДЬЗЧбЁдёЬтдіЗжзЈСЗ гаЛњЭЦЖЯСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЛЏКЯЮяA(C12H16O2)ОМюадЫЎНтЁЂЫсЛЏКѓЕУЕНBКЭC(C8H8O2)ЁЃCжаКЌгаБНЛЗЃЌЧвБНЛЗЩЯга2жжЧтдзгЁЃBОЙ§ЯТСаЗДгІКѓЕУЕНGЃЌGгЩЬМЁЂЧтЁЂбѕШ§жждЊЫизщГЩЃЌЯрЖдЗжзгжЪСПЮЊ172ЃЌдЊЫиЗжЮіБэУїЃЌКЌЬМ55.8%ЃЌКЌЧт7.0 %ЃЌКЫДХЙВеёЧтЦзЯдЪОжЛгавЛИіЗхЁЃ

ЧыЛиД№ЯТСаЮЪЬтЃК
(1)AЕФНсЙЙМђЪНЮЊ_______________ЃЌGЕФЗжзгЪНЮЊ______________ЁЃ
(2)BЕФУћГЦЮЊ_____________ЃЌDжаЙйФмЭХЕФУћГЦЮЊ________________ЁЃ
(3)аДГіFЁњGЕФЛЏбЇЗНГЬЪНЃК________________________________ЃЌИУЗДгІЪєгк_________(ЬюЗДгІРраЭ)ЁЃ
(4)аДГіТњзуЯТСаЬѕМўЕФCЕФ3жжЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК___________________ЁЃ
ЂйЪЧБНЕФЖдЮЛЖўШЁДњЛЏКЯЮяЃЛЂкФмгыFeCl3ШмвКЗЂЩњЯдЩЋЗДгІЃЛЂлВЛПМТЧЯЉДМ( )НсЙЙЁЃ
)НсЙЙЁЃ
(5)дкGЕФДжВњЮяжаЃЌОМьВтКЌгаОлКЯЮядгжЪЁЃаДГіОлКЯЮядгжЪПЩФмЕФНсЙЙМђЪН(аДГі1жжМДПЩ)ЃК_________________________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжГхДЬЗЧбЁдёЬтдіЗжзЈСЗ ЭМЯёЁЂЭМБэСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
1 LФГЛьКЯШмвКЃЌПЩФмКЌгаЕФРызгШчЯТБэЁЃ
ПЩФмДѓСПКЌгаЕФбєРызг | HЃЋЁЂKЃЋЁЂMg2ЃЋЁЂAl3ЃЋЁЂNH4+ЁЂFe2ЃЋЁЂFe3ЃЋ |
ПЩФмДѓСПКЌгаЕФвѕРызг | ClЃЁЂBrЃЁЂIЃЁЂCO32ЁЊЁЂAlO2ЁЊ |
(1)ЭљИУШмвКжаж№ЕЮМгШыNaOHШмвКЃЌВњЩњГСЕэЕФЮяжЪЕФСП(n)гыМгШыNaOHШмвКЕФЬхЛ§(V)ЕФЙиЯЕШчЭМЫљЪОЁЃдђИУШмвКжавЛЖЈВЛКЌгаЕФРызгЪЧ_________________________ЁЃ

(2)BCЖЮРызгЗНГЬЪНЮЊ_______________________________________________ЁЃ
(3)V1ЁЂV2ЁЂV3ЁЂV4жЎМфЕФЙиЯЕЮЊ__________________________________________ЁЃ
(4)ОМьВтЃЌИУШмвКжаЛЙКЌгаДѓСПЕФClЃЁЂBrЃЁЂIЃЃЌШєЯђ1 LИУЛьКЯШмвКжаЭЈШывЛЖЈСПЕФCl2ЃЌШмвКжаClЃЁЂBrЃЁЂIЃЕФЮяжЪЕФСПгыЭЈШыCl2ЕФЬхЛ§(БъзМзДПі)ЕФЙиЯЕШчБэЫљЪОЃЌЗжЮіКѓЛиД№ЯТСаЮЪЬтЁЃ
Cl2ЕФЬхЛ§(БъзМзДПі) | 2.8 L | 5.6 L | 11.2 L |
n(ClЃ) | 1.25 mol | 1.5 mol | 2 mol |
n(BrЃ) | 1.5 mol | 1.4 mol | 0.9 mol |
n(IЃ) | a mol | 0 | 0 |
ЂйЕБЭЈШыCl2ЕФЬхЛ§ЮЊ2.8 LЪБЃЌШмвКжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ_________________ЁЃ
ЂкдШмвКжаClЃЁЂBrЃЁЂIЃЕФЮяжЪЕФСПХЈЖШжЎБШЮЊ_____________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжГхДЬЗЧбЁдёЬтдіЗжзЈСЗ ЛЏбЇЙЄвеСїГЬСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЙЄвЕЬМЫсФЦ(ДПЖШдМЮЊ98%)жаКЌгаCa2ЃЋЁЂMg2ЃЋЁЂFe3ЃЋЁЂClЃКЭSO42ЁЊЕШдгжЪЃЌЬсДПЙЄвеСїГЬШчЯТЃК

Ђё.ЬМЫсФЦЕФБЅКЭШмвКдкВЛЭЌЮТЖШЯТЮіГіЕФШмжЪШчЯТЭМЫљЪОЃК

Ђђ.гаЙиЮяжЪЕФШмЖШЛ§ШчЯТЃК
ЮяжЪ | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
Ksp | 4.96ЁС10Ѓ9 | 6.82ЁС10Ѓ6 | 4.68ЁС10Ѓ6 | 5.61ЁС10Ѓ12 | 2.64ЁС10Ѓ39 |
ЛиД№ЯТСаЮЪЬтЃК
(1)МгШыNaOHШмвККѓЙ§ТЫЕУЕНЕФТЫдќжажївЊКЌга________(ЬюаДЛЏбЇЪН)ЁЃ25ЁцЪБЃЌЯђКЌгаMg2ЃЋЁЂFe3ЃЋЕФШмвКжаЕЮМгNaOHШмвКЃЌЕБСНжжГСЕэЙВДцЧвШмвКЕФpHЃН8 ЪБЃЌc(Mg2ЃЋ)ЁУc(Fe3ЃЋ)ЃН________ЁЃ
(2)ВйзїXЮЊ________ЃЌЦфЮТЖШгІПижЦдк_____________________________________
(3)гаШЫДгЁАТЬЩЋЛЏбЇЁБНЧЖШЩшЯыНЋЁАФИвКЁБбиСїГЬжаащЯпЫљЪОНјаабЛЗЪЙгУЁЃЧыФуЗжЮіЪЕМЪЙЄвЕЩњВњжаЪЧЗёПЩаа________ЃЌВЂЫЕУїРэгЩ______________________________
________________________________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжГхДЬбЁдёЬтЯоЪБЬсЫйзЈСЗ ЖЈСПЗжЮіСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЩшNAЮЊАЂЗќМгЕТТоГЃЪ§ЕФжЕЃЌЯТСаЗЈЫЕжае§ШЗЕФЪЧ(ЁЁЁЁ)
AЃЎ25 ЁцЪБЃЌpHЃН13ЕФ1.0 L Ba(OH)2ШмвКжаКЌгаЕФOHЃЪ§ФПЮЊ0.2 NA
BЃЎБъзМзДПіЯТЃЌ2.24 L Cl2гызуСПЕФH2OЗДгІЃЌзЊвЦЕФЕчзгзмЪ§ЮЊ0.1 NA
CЃЎБъзМзДПіЯТЃЌ22.4 LМзДМ(CH3OH)жаКЌгаЕФбѕдзгЪ§ЮЊ1.0 NA
DЃЎ4.6 g NaдкПеЦјжаЭъШЋЗДгІЩњГЩNa2OЁЂNa2O2ЃЌзЊвЦ0.2 NAИіЕчзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИпПМЛЏбЇЖўТжзЈЬтИДЯА ЕчЛЏбЇСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЙигкЯТСаЫЕЗЈжае§ШЗЕФЪЧ(ЁЁЁЁ)
A.гУЖшадЕчМЋЕчНтШлШкТШЛЏФЦЕФРызгЗНГЬЪНЮЊ:2Cl-+2H2O Cl2Ёќ+H2Ёќ+2OH-
Cl2Ёќ+H2Ёќ+2OH-
B.гУЖшадЕчМЋЗжБ№ЕчНтCuCl2ШмвККЭMgCl2ШмвКЗжБ№ЕУЕНЕЅжЪCuКЭMg
C.ЯТЭМЮЊЕчНтзуСПТШЛЏФЦШмвКЕФзАжУ,вЛЖЮЪБМфКѓСНМЋЪеМЏЕНЦјЬхЕФЬхЛ§жЎБШЮЊ1ЁУ1

D.ЕчНтОЋСЖЭЪБ,ЯрЭЌЪБМфФквѕМЋЮіГіЭЕФжЪСПБШбєМЋШмНтЭЕФжЪСПДѓ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com