
����Ŀ��ij�л�������A�����ϣ��������к�̼Ϊ70.59%������Ϊ 5.88%,���ຬ�������������з����ⶨ���л����������Է��������ͷ��ӽṹ��
����һ:������������֪A������ͼ���£�
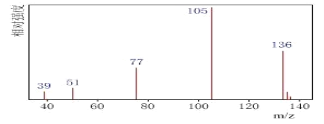
������:�˴Ź����Dz��A�ĺ˴Ź���������4����,�����֮��Ϊ1��1��1��3����ͼA��
������:���ú�������Dz��A���ӵĺ������,��ͼB��
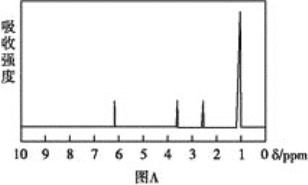
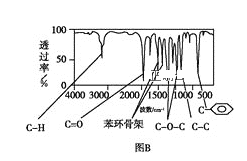
(1)�����й���____�ֻ�ѧ������ͬ����ԭ�ӡ�
(2)A�ķ���ʽΪ____��
(3)������������һ���л���____��
(4)A�ķ�����ֻ��һ������������____(�����)��
a.A����Է������� b.A�ķ���ʽ
c.A�ĺ˴Ź�������ͼ d .A���ӵĺ������ͼ
(5)A�Ľṹ��ʽΪ__________________________________________��
(6)A�ķ�����ͬ���칹���ж���,������ͬʱ������������:����������;�ڷ��ӽṹ�к���һ��������÷�����A��ͬ���칹�干��____�֡��ṹ��ʽΪ(�ٳ�����һ��)______________.
(7)C4H4�ڹ�ҵ���Ǻ���Ҫ��ϩȲ������������Ʊ��ϳ��ĵ���2-�ȶ���ȼ-[1��3]�ȡ����ж���ͬ���칹�壬��������������д����Ӧͬ���칹��Ľṹ��ʽ��
��AΪ��״�ṹ��������������Ȳ�ӳɶ��ã���AΪ___________��
��BΪ��������ṹ��ÿ��̼ԭ�ӷֱ�������3��̼ԭ��ͨ�����������ӣ���B�Ľṹ��ʽΪ___________��
������ڢ���B���ʵĽṹ��ʽ����д��C8H8��һ�ֽṹ��ʽ��Ҫ��ÿ��̼ԭ�ӷֱ���������̼ԭ��ͨ�����������ӡ���ṹ��ʽΪ______________.
���𰸡�4 C8H8O2 ���� bc ![]() 4
4 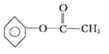 ����
����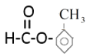 ��
��![]() ��
��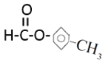 �� CH2=CH��C��CH
�� CH2=CH��C��CH 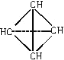
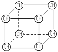
��������
����A�ĺ˴Ź������������շ���Ŀ�ж��京�еĵ�Ч��ԭ����Ŀ������A����Է���������A������C��H����������ȷ��A�ķ���ʽ������ͼBȷ��A�����к��еĹ����ţ�Ȼ���ж������ͣ�����A�ķ���ʽ����˴Ź���������ȷ��A�����к���1������
�������Ϸ���ȷ��A�Ľṹ��ʽ��
(1)����A�ĺ˴Ź�������֪��A��4���壬��A����4����ԭ�ӣ�
(2)A������C��H��Oԭ����Ŀ֮��Ϊ��![]() ����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ(C4H4O)n����Mr(A)=68n=136����ã�n=2�������ʽΪC8H8O2��
����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ(C4H4O)n����Mr(A)=68n=136����ã�n=2�������ʽΪC8H8O2��
�ʴ�Ϊ��C8H8O2��
(3)��ͼB֪��A���б�����ռ6��Cԭ�ӣ�������C=O��COC��CC��CH������C=O��COC�����Ϊ![]() �����Ը�����Ϊ���ࣻ
�����Ը�����Ϊ���ࣻ
�ʴ�Ϊ�����ࣻ
(4)�������(3)�Ľ������ٽ��ͼA����4����ԭ�ӣ��������Ϊ1:1:1:3��������CH3����ԭ����Ϊ3������ֻ����һ��CH3������A�ķ�����ֻ��һ����������Ϊbc��
�ʴ�Ϊ��bc��
(5)�������Ϸ�����֪��A�Ľṹ��ʽΪ��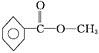 ��
��
(6)A�ķ�����ͬ���칹���ж��֣�������ͬʱ�����������������������ࣻ�����ӽṹ�к���һ��������ṹ��ʽ�У� ��
�� ��
��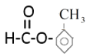 ��
��![]() ��
�� ����ȥA������ͬ���칹�������Ϊ4�֣�
����ȥA������ͬ���칹�������Ϊ4�֣�
�ʴ�Ϊ��4�� ����
����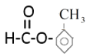 ��
��![]() ��
�� ����
����
(7)�����뵽H2O��CH2=CH2�ӳ�ʱ��H2O�����ֻ���H������OH�ֱ�ӵ���ϩ����������̼ԭ���ϣ���֪��������Ȳ�ӳ�ʱ����һ����Ȳ�����е����ֻ���H����![]() �ֱ�ӵ���һ����Ȳ�����е�����̼ԭ���ϣ���A�Ľṹ��ʽΪ��
�ֱ�ӵ���һ����Ȳ�����е�����̼ԭ���ϣ���A�Ľṹ��ʽΪ��![]() �������뵽��������ṹ�����м����ͺͰ��������֣��ٸ��ݸ÷�����ÿ��̼ԭ�ӷֱ�������3��̼ԭ��ͨ��̼̼���������ӣ���֪�÷��ӽṹ����������ƣ�����B�Ľṹ��ʽΪ��
�������뵽��������ṹ�����м����ͺͰ��������֣��ٸ��ݸ÷�����ÿ��̼ԭ�ӷֱ�������3��̼ԭ��ͨ��̼̼���������ӣ���֪�÷��ӽṹ����������ƣ�����B�Ľṹ��ʽΪ��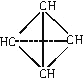 ���۷���ʽΪC8H8��ÿ��̼ԭ�ӷֱ���������̼ԭ��ͨ�����������ӣ�����ṹ��ʽΪ��
���۷���ʽΪC8H8��ÿ��̼ԭ�ӷֱ���������̼ԭ��ͨ�����������ӣ�����ṹ��ʽΪ��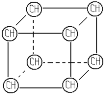 ��
��
�ʴ�Ϊ��![]() ��
�� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������ʽΪC12H26��ij��������Ϊ��2��4��������3��5�����һ�����������һͬѧ��Ϊ���������д������йظ�������˵��������Ϊ��ȷ����
A. ���������ɵ�ϩ���������ӳɶ�������ԭ��ϩ������11�ֲ�ͬ�ṹ
B. ��������һ�ȴ�����10��
C. ��������ȷ����ӦΪ2��4��5��������3���һ�����
D. �����������к���5��֧��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�������������Ʊ���Ѫ����ij�о�С���Ʊ��� FeCO3������ FeCO3 �����ʺ�Ӧ�ý�����̽���� ��֪����FeCO3 �ǰ�ɫ���壬������ˮ��Fe2��+6SCN-![]() Fe(SCN)64-(��ɫ)
Fe(SCN)64-(��ɫ)
��. FeCO3 ����ȡ���г�װ���ԣ�
ʵ��i��
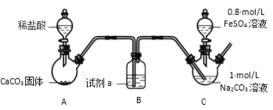
װ�� C �У��� Na2CO3 ��Һ��pH��11.9��ͨ��һ��ʱ�� CO2 ���� pH Ϊ 7���μ�һ���� FeSO4 ��Һ��������ɫ���������ˡ�ϴ�ӡ�����õ� FeCO3 ���塣
(1)�Լ� a ��_____��
(2)�� Na2CO3 ��Һͨ�� CO2 ��Ŀ����_____��
(3)C װ������ȡ FeCO3 �����ӷ���ʽΪ_____��
(4)��ͬѧ��Ϊ C �г��ְ�ɫ����֮��Ӧ����ͨ CO2������Ϊ�Ƿ������˵������________��
��.FeCO3 ������̽��
ʵ��ii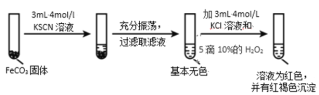
ʵ��iii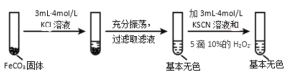
(5)�Ա�ʵ�����������ó���ʵ�������_____��
(6)����ʵ����������д������ 10%H2O2 ��Һ�����ӷ���ʽ_____��
��.FeCO3 ��Ӧ��
(7)FeCO3 ���������CH3CH(OH)COOH�����Ƶÿ���������������[CH3CH(OH)COO]2Fe����Է������� Ϊ 234����Ѫ����Ϊ�ⶨ��Ѫ����������������������������������������������ѧ��ѧʵ��С��ȷ�� �� 1.0g ��Ѫ���������� KMnO4 ��Һ�ζ��ò�Ѫ�������� 0.1000mol/L �� KMnO4 ��Һ 10.00mL�������������ڲ�Ѫ���е���������Ϊ_____������ֵ�쳣��ԭ����________�������Dz� �������Լ��Լ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A.H2(g)+I2(g)![]() 2HI(g)�������������䣬��С��Ӧ������������淴Ӧ���ʲ���
2HI(g)�������������䣬��С��Ӧ������������淴Ӧ���ʲ���
B.C(s)+H2O(g)![]() H2(g)+CO(g)��̼���������ٸı�˵����Ӧ�Ѵ�ƽ��
H2(g)+CO(g)��̼���������ٸı�˵����Ӧ�Ѵ�ƽ��
C.��ѹǿ������ʱ��仯��˵����Ӧ2A(��)+B(g)![]() 2C(��)�Ѵ�ƽ�⣬��A��C����ͬʱ������
2C(��)�Ѵ�ƽ�⣬��A��C����ͬʱ������
D.��ѹ�����·�����ӦN2(g)+3H2(g)![]() 2NH3(g)��������ѹǿ���ٸı�ʱ����Ӧ�ﵽƽ��״̬
2NH3(g)��������ѹǿ���ٸı�ʱ����Ӧ�ﵽƽ��״̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ャҺ��Al(OH)3��MnO2������Na2Cr2O4�����ǵ��������������ʹNa2Cr2O4������ȫ��ˮ������ij�о�С��������Ƶĵ�����װ�ã���ͼ1����ʹ��Һ����ɹ�������ͺ���Ԫ����Һ�����������á�

��������ķ�������õ�����ͼ��ͼ2��ʾ������ͼ�еIJ��ַ�������ͷ�Ӧ����δ��������

(1)��Ӧ�������Լ�NaOH�ĵ���ʽΪ___��B��C�ķ�Ӧ����Ϊ___��C��Al���Ʊ�������Ϊ___��
(2)��С��̽����Ӧ�ڷ�����������D��Ũ�����ϣ������ȣ��ޱ仯��������Cl2���ɣ�����Ӧֹͣ������ʣ�࣬��ʱ�μ����ᣬ�ֲ���Cl2���ɴ��ж�Ӱ��÷�Ӧ��Ч���е�������___������ţ���
a���¶� b��Cl-��Ũ�� c����Һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ӻ�������ȡ�����������ͼ��
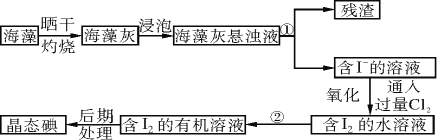
��1��ָ����ȡ��Ĺ������йص�ʵ��������ƣ�����������
�ٲ�������________________��������________________��
��2����ȡ��Ĺ����У��ɹ�ѡ����л��ܼ���________(����ĸ���)��
a���ױ����ƾ� b�����Ȼ�̼����
c�����͡����� d�����͡�����
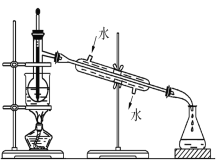
��3��������л�����Һ����ȡ��ͻ����л��ܼ�������Ҫ�������۲�����ʵ��װ��ָ�������֮������ָ����ȷ������____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ��ʱ��ˮ�ĵ���ﵽƽ�⣺H2O![]() H++OH-����H>0������������ȷ����(����)
H++OH-����H>0������������ȷ����(����)
A. ��ˮ�м���ϡ��ˮ��ƽ�������ƶ���c(OH-)����
B. ��ˮ�м������������������ƣ�c(H+)����KW����
C. ��ˮ�м�����������Na��ƽ�������ƶ���c(H+)���ͣ�KW����
D. ��ˮ���ȣ�KW����pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���1L pH��10��NaOH��Һ�г���ͨ��CO2��ͨ���CO2�����(V)����Һ��ˮ��������OH������Ũ��(![]() )�Ĺ�ϵ��ͼ��ʾ������������ȷ����
)�Ĺ�ϵ��ͼ��ʾ������������ȷ����
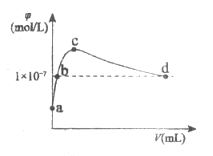
A.a����Һ��ˮ�����c(OH��)��1��10��4mol/L
B.b����Һ��c(OH��)��c(H��)
C.c����Һ��c(Na��)��2c(CO32��)��2c(HCO3��)��2c(H2CO3)
D.d����Һ��c(Na��)��2c(CO32��)��c(HCO3��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯����������������Ӧ�ù㷺���ش��������⣺
(1)���й�����2025�����й�����ʵʩ����ǿ��ս�Ե�һ��ʮ���ж����졣������(CrN)���м��ߵ�Ӳ�Ⱥ���ѧǿ�ȡ�����Ŀ���ʴ���ܺ����ȶ����ܣ����������ִ���ҵ�з��Ӹ���Ҫ�����ã���д��Cr3+����Χ�����Ų�ʽ____����̬������ԭ�ӵĺ���δ�ɶԵ�����֮��Ϊ____��
(2)�������ľ���ṹ�������Ȼ�����ͬ�����������۵�(1282��)���Ȼ��� (801'C)�ĸߣ���Ҫԭ����________��
(3)�������[(NH4)2S2O8]���㷺���������ع�ҵ��ʯ�Ϳ��ɡ����ۼӹ�����֬��ҵ�����ҵ�ȣ����������N��S��O�ĵ�һ�������ɴ�С��˳��Ϊ _______������NH4+�Ŀռ乹��Ϊ____________
(4)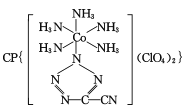 ��20����80����������Ƶĵ��Ͷ۸���ҩ��������
��20����80����������Ƶĵ��Ͷ۸���ҩ��������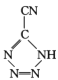 ��[Co(NH3)5H2O](ClO4)3��Ӧ�ϳɵģ�
��[Co(NH3)5H2O](ClO4)3��Ӧ�ϳɵģ�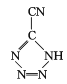 �йµ��Ӷ���������ֵΪ _______�� CP������Co3+����λ��Ϊ ______ ��
�йµ��Ӷ���������ֵΪ _______�� CP������Co3+����λ��Ϊ ______ ��
(5)�����������Ǵ��Բ����о��е��ȵ����֮һ��������и߱��ʹŻ�ǿ�ȡ��ͽ�������������ýϸߵ����ŵ��ʣ����м�����г�DZ���������Ӹ�ṹ��ͼ��ʾ����֪�����ܶ�Ϊ��gcm-3�������ӵ�����ΪNA��
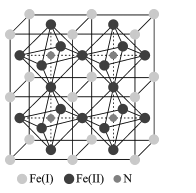
��д�������������Ķѻ���ʽΪ____��
�ڸû�����Ļ�ѧʽΪ ___��
�ۼ���� Fe(II)Χ�ɵİ���������Ϊ____cm3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com