
����Ŀ�������仯����������������Ӧ�ù㷺���ش��������⣺
(1)���й�����2025�����й�����ʵʩ����ǿ��ս�Ե�һ��ʮ���ж����졣������(CrN)���м��ߵ�Ӳ�Ⱥ���ѧǿ�ȡ�����Ŀ���ʴ���ܺ����ȶ����ܣ����������ִ���ҵ�з��Ӹ���Ҫ�����ã���д��Cr3+����Χ�����Ų�ʽ____����̬������ԭ�ӵĺ���δ�ɶԵ�����֮��Ϊ____��
(2)�������ľ���ṹ�������Ȼ�����ͬ�����������۵�(1282��)���Ȼ��� (801'C)�ĸߣ���Ҫԭ����________��
(3)�������[(NH4)2S2O8]���㷺���������ع�ҵ��ʯ�Ϳ��ɡ����ۼӹ�����֬��ҵ�����ҵ�ȣ����������N��S��O�ĵ�һ�������ɴ�С��˳��Ϊ _______������NH4+�Ŀռ乹��Ϊ____________
(4)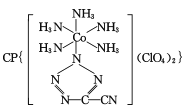 ��20����80����������Ƶĵ��Ͷ۸���ҩ��������
��20����80����������Ƶĵ��Ͷ۸���ҩ��������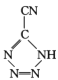 ��[Co(NH3)5H2O](ClO4)3��Ӧ�ϳɵģ�
��[Co(NH3)5H2O](ClO4)3��Ӧ�ϳɵģ� �йµ��Ӷ���������ֵΪ _______�� CP������Co3+����λ��Ϊ ______ ��
�йµ��Ӷ���������ֵΪ _______�� CP������Co3+����λ��Ϊ ______ ��
(5)�����������Ǵ��Բ����о��е��ȵ����֮һ��������и߱��ʹŻ�ǿ�ȡ��ͽ�������������ýϸߵ����ŵ��ʣ����м�����г�DZ���������Ӹ�ṹ��ͼ��ʾ����֪�����ܶ�Ϊ��gcm-3�������ӵ�����ΪNA��
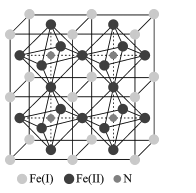
��д�������������Ķѻ���ʽΪ____��
�ڸû�����Ļ�ѧʽΪ ___��
�ۼ���� Fe(II)Χ�ɵİ���������Ϊ____cm3��
���𰸡�3d3 2:1 ����������������������࣬�����ܽϴ� N>O>S �������� 5:4 6 �����������ܶѻ� Fe4N ![]()
��������
(1)��̬Cr�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1����̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3��
(2)����ṹ�������Ȼ�����ͬ���������������Խ�࣬����Խ������Խ���۷е�Խ�ߣ�
(3)ͬ����Ԫ�أ���һ��������˵�������������ͬ����Ԫ�أ���һ��������˵�����������С����Ԫ�����������Ų�����ȫ����������ȫ��ʱ�ṹ�ȶ�����һ�����ܱ�����Ԫ�ظߣ������ӻ������жϿռ乹�ͣ�
(4)˫���к���һ��������һ��������������һ���������������������ݽṹ��ʽ��Ԫ��������������Ԫ�صļ�����ʽ�жϹµ��Ӷ����������������������ֱ������������ĸ�����Ϊ��λ����
(5) ������ͼʾ�����������λ��Ϊ��ԭ�ӣ�
�ڸ���ͼʾ��һ�������е�ԭ�Ӹ����þ�̯�����м��㣻
�۸�����=![]() �Ƶ����㾧���ⳤ���ٸ�����������Ľṹ�ص���������
�Ƶ����㾧���ⳤ���ٸ�����������Ľṹ�ص���������
(1)��̬Cr�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1����6��δ�ɶԵĵ��ӣ���̬Cr3+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d3��Cr3+����Χ�����Ų�ʽ3d3����̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3��3��δ�ɶԵĵ��ӣ���̬������ԭ�ӵĺ���δ�ɶԵ�����֮��Ϊ2:1��
(2)����ṹ�������Ȼ�����ͬ���������������Խ�࣬����Խ������Խ���۷е�Խ�ߣ�����������������������࣬�����ܽϴʵ������۵���Ȼ��Ƶĸߣ�
(3)ͬ����Ԫ�أ���һ��������˵�����������С����S��Oͬ����Ԫ�أ���һ��������˵�������������Ԫ�����������Ų�����ȫ����������ȫ��ʱ�ṹ�ȶ�����һ�����ܱ�����Ԫ�ظߣ���̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3�����ȶ�״̬�����һ������N��O����һ�������ɴ�С��˳��ΪN��O��S��NH4+������ԭ��ΪN����۲���Ӷ���=4+![]() ��(5-1-4��1)=4��Ϊsp3�ӻ����ռ乹��Ϊ�������壻
��(5-1-4��1)=4��Ϊsp3�ӻ����ռ乹��Ϊ�������壻
(4)˫���к���һ��������һ��������������һ������������������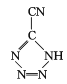 �У���һ��-C��N������2������������˫���зֱ���һ�����������ݽṹ�м�����ʽ��̼ԭ��û�й¶Ե��ӣ�ÿ����ԭ����һ�Թ¶Ե��ӣ��µ��Ӷ���������ֵΪ5:4�������������������ֱ������������ĸ�����Ϊ��λ����CP������Co3+����λ��Ϊ6��
�У���һ��-C��N������2������������˫���зֱ���һ�����������ݽṹ�м�����ʽ��̼ԭ��û�й¶Ե��ӣ�ÿ����ԭ����һ�Թ¶Ե��ӣ��µ��Ӷ���������ֵΪ5:4�������������������ֱ������������ĸ�����Ϊ��λ����CP������Co3+����λ��Ϊ6��
(5)�ٸ���ͼʾ�����������λ��Ϊ��ԭ�ӣ������������Ķѻ���ʽΪ�����������ܶѻ���
�ڸ���ͼʾ��һ�������е���ԭ��Ϊ��������ģ�����=8��![]() +6��
+6��![]() =4����ԭ��λ�ھ����ڲ���N����Ϊ1����û�����Ļ�ѧʽΪFe4N��
=4����ԭ��λ�ھ����ڲ���N����Ϊ1����û�����Ļ�ѧʽΪFe4N��
�۸�����=![]() ���������V=
���������V=![]() �������ⳤ=
�������ⳤ=![]() =
= =
=![]() ��Fe(II)Χ�ɵİ������У��ⳤ=
��Fe(II)Χ�ɵİ������У��ⳤ=![]() ��
��![]() ����������һ������=
����������һ������=![]() ��(
��(![]() ��
��![]() )2��
)2��![]() ��
��![]() =
=![]() ������������Ϊ=2��
������������Ϊ=2��![]() =
=![]() ��
��
![]()


 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л�������A�����ϣ��������к�̼Ϊ70.59%������Ϊ 5.88%,���ຬ�������������з����ⶨ���л����������Է��������ͷ��ӽṹ��
����һ:������������֪A������ͼ���£�
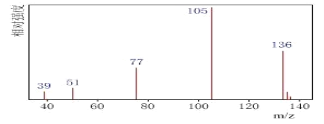
������:�˴Ź����Dz��A�ĺ˴Ź���������4����,�����֮��Ϊ1��1��1��3����ͼA��
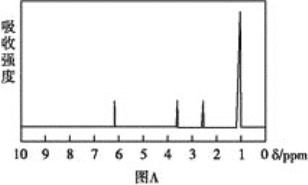
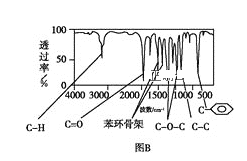
������:���ú�������Dz��A���ӵĺ������,��ͼB��
(1)�����й���____�ֻ�ѧ������ͬ����ԭ�ӡ�
(2)A�ķ���ʽΪ____��
(3)������������һ���л���____��
(4)A�ķ�����ֻ��һ������������____(�����)��
a.A����Է������� b.A�ķ���ʽ
c.A�ĺ˴Ź�������ͼ d .A���ӵĺ������ͼ
(5)A�Ľṹ��ʽΪ__________________________________________��
(6)A�ķ�����ͬ���칹���ж���,������ͬʱ������������:����������;�ڷ��ӽṹ�к���һ��������÷�����A��ͬ���칹�干��____�֡��ṹ��ʽΪ(�ٳ�����һ��)______________.
(7)C4H4�ڹ�ҵ���Ǻ���Ҫ��ϩȲ������������Ʊ��ϳ��ĵ���2-�ȶ���ȼ-[1��3]�ȡ����ж���ͬ���칹�壬��������������д����Ӧͬ���칹��Ľṹ��ʽ��
��AΪ��״�ṹ��������������Ȳ�ӳɶ��ã���AΪ___________��
��BΪ��������ṹ��ÿ��̼ԭ�ӷֱ�������3��̼ԭ��ͨ�����������ӣ���B�Ľṹ��ʽΪ___________��
������ڢ���B���ʵĽṹ��ʽ����д��C8H8��һ�ֽṹ��ʽ��Ҫ��ÿ��̼ԭ�ӷֱ���������̼ԭ��ͨ�����������ӡ���ṹ��ʽΪ______________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2O��NO��NO2�ȵ��������ǿ�����Ⱦ����е��������β���账��������ŷš�
��1��N2O�Ĵ�����N2O�����������а��������ĸ���������ִ�����ʹN2O�ֽ⡣NH3��O2�ڼ��Ⱥʹ�������������N2O�Ļ�ѧ����ʽΪ________��
��2��NO��NO2�Ĵ������ѳ�ȥN2O������β������NaOH��Һ���գ���Ҫ��ӦΪ
NO+NO2+2OH![]() 2
2![]() +H2O
+H2O
2NO2+2OH![]()
![]() +
+![]() +H2O
+H2O
�����д�ʩ�����β����NO��NO2ȥ���ʵ���________������ĸ����
A���ӿ�ͨ��β��������
B����������Һ�����ķ�ʽ����β��
C������β�������ж��ڲ�������NaOH��Һ
�����պ����Һ��Ũ�����ᾧ�����ˣ��õ�NaNO2���壬�þ����е���Ҫ������________���ѧʽ�������պ��ŷŵ�β���к����ϸߵĵ���������________���ѧʽ����
��3��NO���������ա���NaClO��Һ��������β���������β����NO��ȥ���ʡ�����������ͬ��NOת��Ϊ![]() ��ת������NaClO��Һ��ʼpH����ϡ������ڣ��ı仯��ͼ��ʾ��
��ת������NaClO��Һ��ʼpH����ϡ������ڣ��ı仯��ͼ��ʾ��
��������NaClO��Һ�У�HClO����NO����Cl��![]() �������ӷ���ʽΪ________��
�������ӷ���ʽΪ________��
��NaClO��Һ�ij�ʼpHԽС��NOת����Խ�ߡ���ԭ����________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ģ�������N������ʹ������ҩ�����ã���ϳ�·�����£�

��1��A��ϵͳ����Ϊ____________��E�й����ŵ�����Ϊ____________��
��2��A��B�ķ�Ӧ����Ϊ____________���ӷ�Ӧ����Һ̬�л���������ᴿB�ij��÷���Ϊ____________��
��3��C��D�Ļ�ѧ����ʽΪ________________________��
��4��C��ͬ���칹��W�������������칹���ɷ���������Ӧ����1 mol W�����2 mol NaOH������Ӧ������֮һ�ɱ������ɶ�Ԫȩ����������������W��____________�֣���W�ĺ˴Ź�������������壬����ṹ��ʽΪ____________��
��5��F��G�Ĺ�ϵΪ������ţ�____________��
a��̼���칹 b���������칹 c��˳���칹 d��λ���칹
��6��M�Ľṹ��ʽΪ____________��
��7�����������ϳ�·�ߣ���![]() Ϊԭ�ϣ��������·����Ʊ�ҽҩ�м���
Ϊԭ�ϣ��������·����Ʊ�ҽҩ�м���![]() ��
��

��·�����Լ�������1Ϊ____________��X�Ľṹ��ʽΪ____________��
�Լ�������2Ϊ____________��Y�Ľṹ��ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��Ԫ��H2MO4��ˮ�е��������������H2MO4H++HMO4-��HMO4-H++MO42-����������20 mL0.1mol/L NaHMO4��Һ�е���cmol/LNaOH��Һ����Һ�¶������NaOH��Һ�����ϵ��ͼ������˵����ȷ����
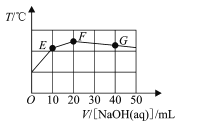
A.������������ҺpH=12
B.ͼ����F���Ӧ����Һ��c(OH-)>c(HMO4-)
C.����NaOH��Һ������ˮ�ĵ���̶�һֱ����
D.ͼ����G���Ӧ����Һ��c(Na+)=c(HMO4-)+2c(MO42-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����о���Ա������ʵ���з���ҩ�������Τ���¹ڲ����������������á�E�Ǻϳ������Τ���м��壬��ϳ�·�����£�
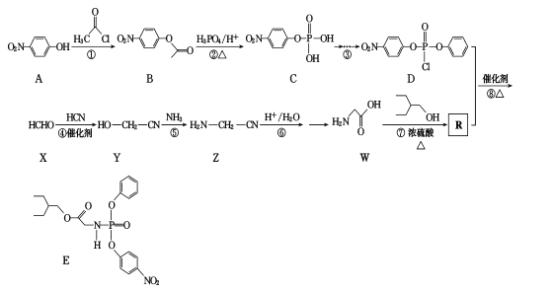
�ش��������⣺
(1)W�Ļ�ѧ����Ϊ____����Ӧ�ٵķ�Ӧ����Ϊ____
(2)A�к��������ŵ�����Ϊ____��
(3)д����Ӧ�ߵĻ�ѧ����ʽ_____
(4)��������������B��ͬ���칹����____��(�����������칹)��
�ٱ��Ķ�ȡ�����ұ����Ϻ����������ڿ��Է���ˮ�ⷴӦ��
����ͬ���칹���к˴Ź�������Ϊ3:2:2�Ľṹ��ʽΪ____________
(5)�л���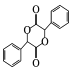 ������̼(��֪��4����ͬ��ԭ�ӻ�ԭ����������̼ԭ�ӳ�Ϊ����̼)�� ___������������Ϣ����ѧ֪ʶ������ɱ��״�Ϊԭ���Ʊ�
������̼(��֪��4����ͬ��ԭ�ӻ�ԭ����������̼ԭ�ӳ�Ϊ����̼)�� ___������������Ϣ����ѧ֪ʶ������ɱ��״�Ϊԭ���Ʊ�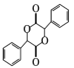 �ĺϳ�·��_______ (���Լ���ѡ)��
�ĺϳ�·��_______ (���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ظ����(K2Cr2O7)��һ����Ҫ�Ļ���ԭ�ϣ���FeO��Cr2O3Ϊԭ���Ʊ�K2Cr2O7���������£�

��֪��2FeO��Cr2O3+4Na2CO3+7NaNO3![]() 4Na2CrO4+Fe2O3+4CO2��+7NaNO2
4Na2CrO4+Fe2O3+4CO2��+7NaNO2
�ش��������⣺
(1)д��������һ����;��___________________��
(2)������pH����ʵ��CrO42-��Cr2O72-����Һ���ת���������£�����ʼŨ��Ϊ1.0 mol��L-1��Na2CrO4��Һ��c(Cr2O72-)��c(H+)�ı仯��ͼ��ʾ��

����ͼ��֪����Һ��������CrO42-��ƽ��ת����________(��������������С������������)��
�ڸ���A�����ݣ��������ת����Ӧ��ƽ�ⳣ��Ϊ_________��
(3)�����е���ת���������з����ķ�Ӧ����_________(�������Ӧ����)��
(4)�ڻ�ѧ�����в���K2CrO4Ϊָʾ������AgNO3����Һ�ζ���Һ�е�Cl-������Ag+��CrO42-����ש��ɫ������ָʾ����ζ��յ㡣����Һ��Cl-ǡ����ȫ����(Ũ�ȵ���1.0��10��5 mol��L-1)ʱ����Һ��c(Ag+)Ϊ_______mol��L-1����ʱ��Һ��c(CrO42-)����________mol��L��1��(��֪Ag2CrO4��AgCl��Ksp�ֱ�Ϊ2.0��10-12��2.0��10-10)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯ�ͷ���õ������ͽ����ѻ����Ի�ý϶������ȼ�͡�ij�о���ѧϰС��ģ�ҵ.��ʯ�͵Ĵ��ѻ��������ͼ��ʾʵ��װ�á�ʵ������пɹ۲쵽��ƿ�й���ʯ�����ۻ����Թ�II��������Һ�����ɣ��Թܹ������Ը��������Һ��ɫ��ʵ������Թ�II��Һ�����ζ��������������͵���ζ��

����һ ʯ���Ǻ���20 ~ 30��̼ԭ�ӵ�������ɵĻ��������³ʹ�̬��
���϶� ʯ�ʹ��ѻ�������ͨ��ʹ��![]() ��������
��������
��1��Ϊ��֤ʵ��ɹ���ʵ��ǰ������еIJ�����_________________��װ���г����ܵ�������________________________��
��2���Թ�II��������Һ������˵��________________��
��3���Թ�III����Һ��ɫ˵��_______________________��
��4��_______����ܡ����ܡ������Թ�II�е�Һ����ȡ��ˮ�е��壬������___________��
��5��д����ʮ���ѻ��õ������ϩ�Ļ�ѧ����ʽ��___________________________��
��6��ʯ���ѻ�����ҪĿ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�鲻�ܴﵽԤ��ʵ��Ŀ���ǣ���
��� | ʵ������ | ʵ��Ŀ�� |
A | �����£���pH��ֽ�ⶨŨ��Ϊ0.1mol��L-1NaClO��Һ��0.1mol��L-1CH3COONa��Һ��pH | �Ƚ�HClO��CH3COOH������ǿ�� |
B | ��ʢ��1mL��������Һ���Թ��еμ�NaCl��Һ���������г������ɣ��������еμ�Na2S��Һ | ˵��һ�ֳ�����ת��Ϊ��һ���ܽ�ȸ�С�ij��� |
C | ��������FeCl3��MgCl2��Һ�м�������Mg(OH)2��ĩ������һ����� | ��ȥMgCl2������FeCl3 |
D | �����£��ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ���ٷֱ������ͬ�����ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com