
��̼����������ָͨ��һ���ķ�������ҵ�����в�����CO2������������á��������NaOH��Һ��������CO2���������������ͼ��ʾ����������������δ�������
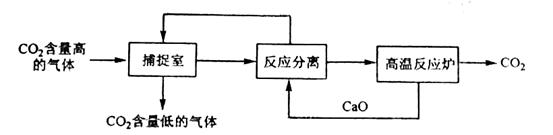
�����йظ÷�������������ȷ����
A.�ܺĴ��Ǹ÷�����һ��ȱ��
B.���������У�ֻ��һ�����ʿ���ѭ������
C.����Ӧ���롱�����У��������ʵĻ��������������ᾧ������
D.�÷����ɼ���̼�ŷţ�������CO2���������Ʊ��״��Ȳ�Ʒ
���𰸡�AD
�������������֪������������������Ӧ���ٶ�����̼���������Ʒ�Ӧ����̼��Ƶĸ��·ֽ⡣Aѡ����ȷ��ѭ�����õ�Ӧ����CaO��NaOH �������ʣ�Bѡ�������Ӧ���롱�����з������ʵIJ���Ӧ���ǹ��ˣ�Cѡ�����Dѡ���м״���ҵ�Ͽ���CO2�Ʊ���
�����ɵ㲦������������Ϣ��֪�������з�ӦΪ������̼���������Ʒ�Ӧ���õ���Na2CO3��CaO����Һ�з�Ӧ�õ�NaOH��CaCO3���ɴ˿ɷ�������ѡ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�Ͼɸɵ���е��������Ҫ�ж������̡�̿�ڡ��Ȼ�п���Ȼ�李����ۺ���Mn2O3��ZnO��FeO�����Ļ�����ȡ�ijʵ��С���ͬѧ����շϾɵ���еijɷ֣���Ҫ������������ͼ��
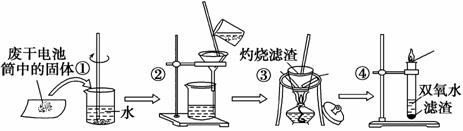
�Իش��������⡣
(1)������ò����������Ŀ����_______________________________________
___________________________________________________________________��
����ڹ��˺���Һ�е���Ҫ������____________(�ѧʽ)������������յ�Ŀ����__________________________________ ______________________________________��
______________________________________��
(2)��������۵õ����Ǵ��ƶ������̣�Ҫ�õ����ƵĶ������̻��轫���ƶ���������ϡ����ϴ�Ӻ�����ˮϴ�ӣ�ϡ�����������___________________________________
______ ______________________________________________________________��
______________________________________________________________��
(3)д��������з�����Ӧ�Ļ�ѧ����ʽ��__________________________________
________________________________________________________________________��
��������ڵõ��Ĺ����������������֮��ϼ��ȵ�ʵ��________(��ܡ����ܡ�)����ʵ��ܡ�
(4)��֪NH4Cl��ZnCl2���ܽ��(g/100 gˮ)���±���
| �¶�(��) | 20 | 30 | 40 | 60 | 80 |
| NH4Cl | 37.2 | 31.4 | 45.8 | 65.6 | 77.3 |
| ZnCl2 | 396 | 437 | 452 | 541 | 614 |
����Һ�з���NH4Cl��ZnCl2�ķ�����___________________________________��
(5)���к����ʱ���������ĽǶȿ��ǣ�������Ʋ������ĵط���___________________________________ _____________________________________��
_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������Mg��Zn��Al�����������ϡH2SO4��Ӧ������H20.125mol��ԭ���������������� �� ��
A��2g B��4g C��8g D��10g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PH3��һ����ɫ�綾���壬����ӽṹ��NH3���ƣ���P−H�����ܱ�N−H�����ܵ͡������жϴ������
A��PH3���ӳ�������
B�� PH3�����Ǽ��Է���
C��PH3�е����NH3�е㣬��ΪP-H�����ܵ�
D��PH3�����ȶ��Ե���NH3���ӣ���ΪN-H�����ܸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ����
A��Һ���ӷ����ڴ��Һ����Լ�ƿ��Ӧ��ˮ��
B����ʹ��ʪ�ĵ���KI��ֽ�����ɫ������һ����Cl2
C��ij��Һ����CCl4��CC14������ɫ��֤��ԭ��Һ�д���I−
D��ij�ܱ�����BaCl2��Һ������������ϡ����İ�ɫ����������Һһ������Ag��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪�������ԣ�
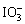 ��Fe3����I2����ԭ�ԣ�
��Fe3����I2����ԭ�ԣ� ��I����
��I����
3I2��6OH��
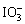 ��5I����3H2O��
��5I����3H2O��
KI��I2 KI3
KI3
��1��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ������_________���û�ѧʽ��ʾ����CCl4�����Ϻ�ɫ��������___________________���õ���ʽ��ʾ����
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ___________________________��______________________________________��
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��
д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_____________________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ�______����ǡ�������˵������________________________________________��
��3��Ϊ����ӵ��Σ�����KI�����ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ�������___________________��
A��Na2S2O3 B��AlCl3 C ��Na2CO3 D��NaNO2
��4���Ժ�Fe2���϶��ʳ��(���費��Fe3��)����ѡ��KI��Ϊ�ӵ���������ʵ�鷽��������üӵ����е�Fe2����__________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ƶ���ȷ����
A��SiO2 ���������������NaOH��Һ��Ӧ
B��Na2O��Na2O2���Ԫ����ͬ���� CO2��Ӧ����Ҳ��ͬ
C��CO��NO�� NO2���Ǵ�����Ⱦ���壬�ڿ����ж����ȶ�����
D��������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯīϩ����̼ԭ�ӹ��ɵĵ���Ƭ״�ṹ���²���(�ṹʾ��ͼ����ͼ),����ʯī�������,���м��õ�Ӧ��ǰ��������˵����ȷ����( )

A.ʯīϩ��ʯī��Ϊͬλ��
B.0.12 gʯīϩ�к�6.02��1022��̼ԭ��
C.ʯīϩ��һ���л���
D.ʯīϩ��̼ԭ�Ӽ��Թ��ۼ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о����֣�������������NO2�ܲ���������������γɣ���Ӧ�������£�
 ��SO2+NO2
��SO2+NO2 SO3+NO
SO3+NO
��SO3+H2O H2SO4
H2SO4
��2NO+O2 2NO2
2NO2
NO2�����������е����ã���H2SO4�������仯�е��������Ƶ��ǣ� ��
A.��ʪ������ͨ��ʢ��ŨH2SO4��ϴ��ƿ B.����ͨ��ŨH2SO4��
C.ŨH2SO4����өʯ�У����� D.��������H2SO4ʹ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com