
ij�����ijɷ�ΪCu2O��Al2O3��Fe2O3��SiO2����ҵ���øÿ�����ȡͭ�͵����IJ����������£�
��֪�� ��Cu2O +2 H+="Cu" + Cu2++H2O
�ڲ���������������������ʽ����ʱ��Һ��pH���±���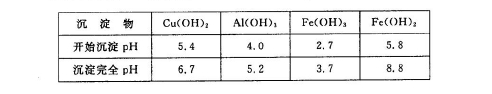
��ش��������⣺
��1��Ϊ�˼ӿ췴ӦI�����ʣ����Բ�ȡ�Ĵ�ʩ�� (д2��)��
��2����������A�еijɷ��� ��
��3����ӦI��ɺ���Ԫ�صĴ�����ʽΪ (�����ӷ���)��д�����ɸ����ӵ����ӷ���ʽ ��
��4������1��Ҫ������ �� �� ��ϴ��CuSO4?5H2O�ֲ�Ʒ�����ô���ˮϴ�����ñ�ˮϴ�ӡ�ԭ���� ��
��5���ö��Ե缫������Һһ��ʱ�䣬����0��1 mol��Cu(OH)2�ɻָ���Һԭ��(Ũ�ȡ��ɷ�)������ʱת�Ƶ��ӵ����ʵ���Ϊ ����
��6����NaClO��pH�����ɳ���B��ͬʱ����һ�־���Ư�����õ����ʣ��÷�Ӧ�����ӷ���ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʽ������[Fe(OH)SO4]��һ��������ˮ����������Ч����������ҵ�����÷���м�����������������������ȣ�������ʽ�������Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ���������ض��dz�����ˮ����������ͼΪ�Ʊ��Ȼ�������һ�������Ʊ�������صĹ������̡�
��ش��������⣺
��1���Ȼ����ж�����;���������ӷ���ʽ��ʾ������;��ԭ����
���Ȼ�������ˮ��______________________��
����FeCl3��Һ��32%��35%����ʴͭӡˢ��·��____________________________��
��2�����ռ�X�Ļ�ѧʽΪ ���� ��������Y�Ļ�ѧʽΪ________________��
��3�����������·�Ӧ�ٵ����ӷ���ʽΪ____________________________________��
��4�����̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ
2KOH��Na2FeO4��K2FeO4��2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��_________��
��5��K2FeO4��ˮ��Һ��������Ӧ��4FeO42-+10H2O 4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
4Fe(OH)3+8OH-+3O2�������ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ������ţ���
| A��H2O | B��ϡKOH��Һ������� | C��NH4Cl��Һ������� | D��Fe(NO3)3��Һ������� |
 CrO42-��Fe(OH)3����OH-
CrO42-��Fe(OH)3����OH- Cr2O72-��H2O
Cr2O72-��H2O 2Cr3����6Fe3����7H2O
2Cr3����6Fe3����7H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��1��26��15ʱ�����й��������;���������ˣ�20�ɹ����䡣��־���ˣ�20��Բ���ɹ�����ش��������⣺
��.�ˣ�20���ݽṹ������Ҫ��ͬ��ʹ�õIJ���Ҳ��ͬ������Ϊ���в����������ˣ�20���죬�������� (�����)��
| A�����ô��������ˣ�20������ |
| B����þ���Ͻ������ˣ�20���� |
| C���������������ˣ�20������·�ĵ��� |
| D������ͨ�������ˣ�20���������� |
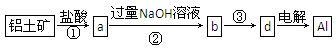
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ի�ͭ��(��Ҫ�ɷ���CuFeS2�����ʲ�����ˮ����)Ϊԭ�ϣ��Ʊ���ɫ����G���仯ѧʽΪ[Cu(NH3)4]SO4��H2O���漰�������£� 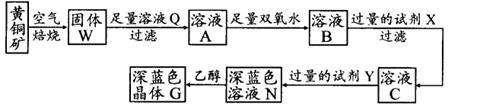
��֪25��ʱ�����ֽ�������������ܶȻ���������ȫ������pH��Χ���±���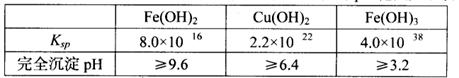
��1����ͭ���ڿ����б�������������ͭ�ĵͼ����д���䷴Ӧ�Ļ�ѧ����ʽ�� ��
��2���Լ�X�Ļ�ѧʽΪ ��
��3�������£�0.1 mol��L�Լ�Y��pH=11������¶��£��Լ�Y�ĵ��볣��Ϊ ����pH��ֽ�����ҺpHֵ�ķ����� ��
��4������ҺN�м����Ҵ���Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬C
Ϊ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ(����ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ)��
(1)д����ѧʽ��A________��D________��E________��X________��
(2)�ڷ�Ӧ�١����У�������������ԭ��Ӧ����________(����)��
(3)��Ӧ�����ӷ���ʽΪ��_______________________________________��
(4)��Ӧ�ߵĻ�ѧ����ʽΪ_________________________________________��
�÷�Ӧ��ÿ����0.3 mol��A����ת�Ƶ���______mol��
(5)д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽ��_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����仯����Ӧ�ù㷺��
��1�����Ȼ�����һ��ˮ����������ҵ�Ʊ���ˮ���Ȼ�������IJ�����������ͼ��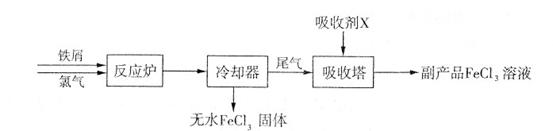
�ټ��鸱��Ʒ�к���Xʱ��ѡ�õ��Լ��� (�����и��������)��
a��NaOH��Һ b��KSCN��Һ c������KMnO4��Һ d������
�����������У����ɸ���ƷFeCl�������ӷ���ʽΪ
��2���������(K2FeO4)Ҳ��һ��������ˮ����������ҵ�ϣ������������������KOH��Һ�Ʊ�������ء��������У������ĵ缫��ӦʽΪ �����һ��ʱ�����������������28 g�����ڴ˹����У����������������ڱ�״���µ����Ϊ L��
��3�������������ڹ�ҵ��ˮ�Ĵ�����
������Ϊ���ܷ���������������Cd2+�Ĺ�ҵ��ˮ? (��ܡ���)������ݳ����ܽ�ƽ���ԭ��������Ĺ۵�(�ñ�Ҫ�����ֺ����ӷ���ʽ˵��)�� (��֪��25��ʱ���ܶȻ�����Ksp(FeS)=6��310-18��Ksp(CdS)=3��610-29)
�ڹ�ҵ�ϴ�����Cd2+��ˮ�����Բ��ü�̼���Ƶķ�������Ӧ���£�2Cd2++2CO32-+H2O=Cd2(OH)2CO3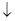 +A����A�Ļ�ѧʽΪ ��
+A����A�Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������һ�ֳ��õĽ������洦������������ʹ���ı�������һ�����ܵ�����Ĥ��������Ĥ������ϡ���ᡣ
ij��ѧ�о�С����ʵ�����а����в���ģ�������桰�ۻ����IJ������̡�
(1)����ʵ���õ���Һ��Ҫ����200 mL�ܶ�Ϊ1.2 g/cm3��������������Ϊ16%��NaOH��Һ����Ҫ��ȡ________g NaOH���塣
(2)����Ƭ�����ȵ�16%��NaOH��Һ��Լ���������ϴȥ���ۣ���ȥ���������Ĥ��ȡ����ˮ��ϴ��д����ȥ����Ĥ�����ӷ���ʽ��________��
(3)����ͼ��װ����������ͨ����K��ͨ��Լ25 min�������������������������������塣
д���ù����еĵ缫��Ӧʽ��
������_________________________________________��
������_________________________________________��
(4)�Ͽ���·��ȡ����Ƭ������������Ϊ1%��ϡ��ˮ�кͱ������Һ������ˮ��ϴ�ɾ���д���ù��̷�����Ӧ�����ӷ���ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�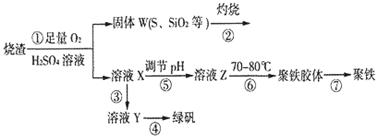
��1�������̢��еIJ���������ͨ��������Һ�У���Һ����ɫ����__________��
A��Ʒ����Һ B����ɫʯ����Һ C������KMnO4��Һ D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ��___________________________________��
��3�����̢��У�������������___________________________��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ��������_______________��
��5�����̢ݵ���pH��ѡ�������Լ��е�___________ (��ѡ�����)��
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70һ80�棬Ŀ����_____________________��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.70g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ___________��(���������в�����Ԫ�غ���Ԫ��)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com