
���ڣ�2C4H10(g)+13O2(g)=8CO2(g)+10H2O(l)����H =��5800kJ/mol�������������
| A���÷�Ӧ�ķ�Ӧ��Ϊ��H=��5800kJ/mol���Ƿ��ȷ�Ӧ |
| B���÷�Ӧ�ġ�H������ʵ�״̬�йأ��뻯ѧ������Ҳ�й� |
| C����ʽ�ĺ���Ϊ��25�桢101kPa�£�2mol C4H10������ȫȼ������CO2��Һ̬ˮʱ�ų�����5800kJ |
| D���÷�ӦΪ����ȼ�յ��Ȼ�ѧ����ʽ���ɴ˿�֪�����ȼ����Ϊ5800kJ/mol |
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����뻯ѧ��Ӧ�����仯��ص���������ȷ����
| A����֪CH4(g)��2O2(g)=CO2(g)��2H2O(g) ��H��-802 kJ/mol�������ȼ����Ϊ802 kJ/mol |
| B������H2��O2����ȫȼ�գ�����H2O(g)������H2O(l)�ų��������� |
| C��ͬ��ͬѹ�£�H2(g)��Cl2(g)=2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��ͬ |
| D����ʯī�Ƚ��ʯ�ȶ���֪��C(���ʯ, s)=C(ʯī, s) ��H<0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и����Ȼ�ѧ����ʽ�У���ѧ��Ӧ�Ħ�Hǰ�ߴ��ں��ߵ��� (����)
��C(s)��O2(g)=CO2(g)����H1 C(s)��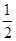 O2(g)=CO(g)����H2
O2(g)=CO(g)����H2
��S(s)��O2(g)=SO2(g)����H3 S(g)��O2(g)=SO2(g)����H4
��H2(g)��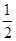 O2(g)=H2O(l)����H5 2H2(g)��O2(g)=2H2O(l)����H6
O2(g)=H2O(l)����H5 2H2(g)��O2(g)=2H2O(l)����H6
��CaCO3(s)=CaO(s)��CO2(g)����H7 CaO(s)��H2O(l)=Ca(OH)2(s)����H8
| A���� | B���� | C���ڢۢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����Ȼ�ѧ����ʽ��ȷ���ǣ�ע��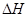 �ľ���ֵ����ȷ��( )
�ľ���ֵ����ȷ��( )
| A��C2H5OH(l)+3O2(g)=2CO2(g) +3H2O(g)����H=" ��1367.0" kJ/mol��ȼ���ȣ� |
| B��NaOH(aq) + HCl(aq)="NaCl(aq)" + H2O(l)����H= ��57.3kJ/mol���к��ȣ� |
| C��S(s) + O2(g) = SO2(g)����H= ��269.8kJ/mol����Ӧ�ȣ� |
| D��2HCl(g)=Cl2(g) + H2(g)����H=" ��" 184.6kJ/mol����Ӧ�ȣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
21�����������ɡ���ʯ��Դʱ����������Դʱ�������ɣ����в���������Դ���ǣ� ��
| A������ | B������ | C��̫���� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���б仯���̣����ڷ��ȷ�Ӧ���ǣ� ��
��Һ̬ˮ���ˮ���� ������кͷ�Ӧ ��ŨH2SO4ϡ��
�ܹ���NaOH����ˮ ��H2��Cl2��ȼ�� ���������
| A���ڢۢܢ� | B���ڢۢ� | C���ڢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪���ȼ��a g��Ȳ(C2H2)����ʱ����1 mol CO2�����Һ̬ˮ�����ų�����b kJ������Ȳȼ�յ��Ȼ�ѧ����ʽ��ȷ����
| A��2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l)��H����4b kJ��mol-1 |
B��C2H2(g)�� O2(g)=2CO2(g)��H2O(l)��H��2b kJ��mol-1 O2(g)=2CO2(g)��H2O(l)��H��2b kJ��mol-1 |
| C��2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l)��H����2b kJ��mol-1 |
| D��2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l)��H����b kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
1������ȼ������Һ̬ˮ�ų�142.9 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽ��ȷ����( )
| A��2H2(g)+O2(g)=2H2O(l)����H="-285.8" kJ/mol |
| B��H2(g)+1/2O2(g)=H2O(g)����H=-142.9kJ/mol |
| C��H2O(l)=H2(g)+1/2O2(g)�� ��H="+285.8" kJ/mol |
| D��2H2+O2=2H2O����H="-571.6" kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ӦA+B��C(��H>0)���������У���A+B��X(��H<0)����X��C(��H>0)������ʾ��ͼ�У�����ȷ��ʾ�ܷ�Ӧ�����������仯����( )
A B C D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com