X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ�
�ش��������⣺
��1��L��Ԫ�ط���Ϊ
O
O
��M��Ԫ�����ڱ��е�λ��Ϊ
�������ڵڢ�A��
�������ڵڢ�A��
������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����
Al��C��N��O��H
Al��C��N��O��H
����Ԫ�ط��ű�ʾ����
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���A��B����֪����̬��B��L�����ĵ�����ȼ������Z���ʺ�ˮ����ʱ�ų�534KJ��������д���÷�Ӧ�Ȼ�ѧ����ʽ
N2H4��g��+O2��g���TN2��g��+2H2O��g��?��H=-534kJ/mol
N2H4��g��+O2��g���TN2��g��+2H2O��g��?��H=-534kJ/mol
��3������se��������������Ԫ�أ���Lͬһ���壬Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��Se������������Ӧ��ˮ���ﻯѧʽΪ
H2SeO4
H2SeO4
������2��5����Ԫ�ص��ʷֱ���H
2��Ӧ����1 mol��̬�⻯��ķ�Ӧ�����£���ʾ����1mol�����ⷴӦ�ȵ���
b
b
������ĸ���ţ���
a��+99.7mol?L
-1 b��+29.7mol?L
-1c��-20.6mol?L
-1 d��-241.8kJ?mol
-1��4����M������������ʯī��������NaHCO
3��Һ�����Һ���е�⣬����������R��ij���壬��֪R���ȷֽ����ɻ�����Q��д����������R�ĵ缫��Ӧʽ��
Al-3e-=Al3+��Al3++3HCO3-=Al��OH��3��+CO2��
Al-3e-=Al3+��Al3++3HCO3-=Al��OH��3��+CO2��
����R����Q�Ļ�ѧ����ʽ��
��

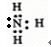
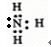




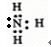 ��N2H4�ĽṹʽΪ��
��N2H4�ĽṹʽΪ�� ��
��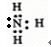 ��
�� ��
�� ��������������Ӧ��ˮ���ﻯѧʽΪH2SeO4���ʴ�Ϊ��34��
��������������Ӧ��ˮ���ﻯѧʽΪH2SeO4���ʴ�Ϊ��34�� ��H2SeO4��
��H2SeO4��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�





