
����Ŀ����������װ��(�гֺͼ�����������ȥ)��ⱥ��ʳ��ˮ�������������������ԣ�ͬʱ�õ�������H2��ԭCuO��ĩ���ⶨCu�����ԭ��������

��1��д����ⱥ��ʳ��ˮ�����ӷ���ʽ_________________________________��
��2��Ϊ�������ʵ�飬��ȷ������˳��ΪA��_______��B��_______ (��д���ܿ���ĸ)��
��3�������������������ԣ�����װ�õ�aƿ����Һ�����������Լ��е�___________��
a.���Ը��������Һ b.���۵⻯����Һ c.����������Һ d.�Ȼ�������Һ
��4����װ�õ�cƿ��ʢ�ŵ��Լ�Ϊ___________��������______________________��
��5��Ϊ�ⶨCu�����ԭ����������������¼ס�������ʵ�鷽����
��ȷ����Ӳ�ʲ����ܵ�����Ϊa g������CuO��ȷ����Ӳ�ʲ����ܺ�CuO��������Ϊb g������CuO��ַ�Ӧ����ʵ����Ϻ�
������ͨ����ȷ����Ӳ�ʲ����ܺ�Cu�۵�������Ϊc g������ȷ��Cu�����ԭ��������
�ҷ�����ͨ����ȷ�ⶨU��b��Ӧǰ��������仯���õ�����ˮ������d g������ȷ��Cu�����ԭ��������
������������ش�___________������������ȷ���������������ⶨ�����ݼ��㣬Cu�����ԭ������Ϊ________________��
�ڲ������ķ�������ɲⶨ���___________���ƫ�͡�ƫ����Ӱ�족����
���𰸡� 2Cl��+2H2O![]() 2OH��+H2��+Cl2�� E C bd Ũ���� ����H2�е�H2O����ֹӲ�ʲ�����ը�ѣ���Ӱ��ⶨˮ����������Ϊ�ҷ����Dz�ˮ�������� �� 16(c��a)/(b��c) ƫ��
2OH��+H2��+Cl2�� E C bd Ũ���� ����H2�е�H2O����ֹӲ�ʲ�����ը�ѣ���Ӱ��ⶨˮ����������Ϊ�ҷ����Dz�ˮ�������� �� 16(c��a)/(b��c) ƫ��
�����������⿼��ʵ�鷽����������ۣ���1����ⱥ��ʳ��ˮ�õ��������������������ƣ������ӷ�Ӧ����ʽΪ��2Cl��+2H2O![]() 2OH��+H2��+Cl2������2�����ݣ�1����A�г���������Ϊ������B�г���������ΪCl2������ʵ��Ŀ�ģ��Լ����⣨5�������A����E��B����C����3�������������������ԣ���Ҫ������ǻ�ԭ����a�����Ը��������Һ������ǿ�����ԣ���a����b��I�����л�ԭ�ԣ�����Cl2��2I��=I2��2Cl������Һ����ɫ��˵��Cl2���������ԣ���b��ȷ��c����ȻNa2SO3���л�ԭ�ԣ���ʵ�����������ж��Ƿ�����Ӧ����c����d��Fe2�����л�ԭ�ԣ�����2Fe2����Cl2=2Fe3����2Cl������Һ��ɫ��dz��ɫ��Ϊ��ɫ��˵��������Ӧ����d��ȷ����4���ͱ�����������ԭ����ͭ�����ⶨCu�����ԭ��������A�г����������л���ˮ������Ӱ��ⶨˮ�����������c������������H2��H2O��Ӧʢ�ŵ��Լ���Ũ�����5�����ҷ�����b�������ͨ�������к���ˮ��������bװ�����գ����ײ�������˼�����������ȷ����������CuO��H2
2OH��+H2��+Cl2������2�����ݣ�1����A�г���������Ϊ������B�г���������ΪCl2������ʵ��Ŀ�ģ��Լ����⣨5�������A����E��B����C����3�������������������ԣ���Ҫ������ǻ�ԭ����a�����Ը��������Һ������ǿ�����ԣ���a����b��I�����л�ԭ�ԣ�����Cl2��2I��=I2��2Cl������Һ����ɫ��˵��Cl2���������ԣ���b��ȷ��c����ȻNa2SO3���л�ԭ�ԣ���ʵ�����������ж��Ƿ�����Ӧ����c����d��Fe2�����л�ԭ�ԣ�����2Fe2����Cl2=2Fe3����2Cl������Һ��ɫ��dz��ɫ��Ϊ��ɫ��˵��������Ӧ����d��ȷ����4���ͱ�����������ԭ����ͭ�����ⶨCu�����ԭ��������A�г����������л���ˮ������Ӱ��ⶨˮ�����������c������������H2��H2O��Ӧʢ�ŵ��Լ���Ũ�����5�����ҷ�����b�������ͨ�������к���ˮ��������bװ�����գ����ײ�������˼�����������ȷ����������CuO��H2![]() Cu��H2O��CuO������Ϊ(b��a)g������Cu������Ϊ(c��a)g������Cuԭ���غ㣬�����(b��a)/(M��16)=(c��a)/M�����M=16(c��a)/(b��c)������ʵ�鷽���ó�Cu�����ԭ������Ϊ[18(b��a)/d]��16��b�������ͨ�������к���ˮ��������bװ�����գ�d���������ӣ�[18(b��a)/d]��16ƫ�͡�
Cu��H2O��CuO������Ϊ(b��a)g������Cu������Ϊ(c��a)g������Cuԭ���غ㣬�����(b��a)/(M��16)=(c��a)/M�����M=16(c��a)/(b��c)������ʵ�鷽���ó�Cu�����ԭ������Ϊ[18(b��a)/d]��16��b�������ͨ�������к���ˮ��������bװ�����գ�d���������ӣ�[18(b��a)/d]��16ƫ�͡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������X�Ļ�ѧʽΪABO3����֪��Ԫ�����ڱ��У�A��B��Ϊǰ����������Ԫ�أ���Aλ��B����һ���ڡ�
��1����������A��B�ĵ��ʶ�����ˮ������Ӧ��
��B��Ԫ�����ڱ��е�λ����___________________��
������˵����ȷ����__________(�����)��
a��A�ļ����Ӱ뾶��B�ļ����Ӱ뾶��
b��A��BԪ�ص�����������Ӧ��ˮ���ﶼ��ǿ�����
c��A������ˮ��Ӧ����Һ��pH��B������ˮ��Ӧ����Һ��pH��
��400��ʱ��X�ܷ����ֽⷴӦ���������Σ������ʵ���֮��Ϊ1��3������һ�����������Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��2����X������ˮ���ڿ��������������ʣ�BԪ��ԭ�ӵ�����������������Ӳ�����2����
��X�ܿ�����������ˮ�е�C1O-���÷�Ӧ�����ӷ���ʽΪ_______________________��
���������ʵ����֤X�Ƿ���ʣ�___________________________________________��
��3����X����ϡ���ᷴӦ��������ɫ����ζ�����塣
�ٸ�����ĵ���ʽΪ______________��
��X��ˮ�г������ȣ�����ˮ�ⷴӦ������һ�ָ����ܵ����ʲ��ݳ����壬��Ӧ�Ļ�ѧ����ʽΪ________________________________________________��
��X����������Ϳ�㣬��ԭ���ǣ�a��������X�����ֽⷴӦʱ�����մ������ȣ�b��______________________________(��дһ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������

A. ��ͼ�����жϣ����ڷ�ӦA(g)��B(g) ![]() 2C(g)����T1>T2������H<0
2C(g)����T1>T2������H<0
B. ͼ�ұ�ʾ���淴ӦCO(g)��H2O(g) ![]() CO2(g)��H2(g)����H>0
CO2(g)��H2(g)����H>0
C. ͼ����ʾCO2ͨ�뱥��Na2CO3��Һ�У���Һ�����Ա仯
D. ͼ����ʾ0.1 mol��L��1������ζ�20 mL 0.1 mol��L��1 NaOH��Һ����ҺpH�������������ı仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һЩ�������ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʱ���ȥ�����³�ѹ�£�AΪ��ɫ�ж����壬BΪ����ɫ��ĩ��C��EΪ�������ʣ�G��J����ɫ��Ϊ��ɫ����Ӧ��Ϊ������¯�е���Ҫ��Ӧ����Ӧ�ڿ����ں������죮
��ش��������⣺
��1��B�Ļ�ѧʽΪ �� J�Ļ�ѧʽΪ
��2����ҵ�Ͻ�����ͨ��ʯ��������ȡƯ�ۣ��÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ
��3��D��G�Ǻ����Ƽ����Ҫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��K��H������ӡˢ��·�����Ҫ��Ӧ���÷�Ӧ�����ӷ���ʽΪ ��
��5��F��L���������ᴿ�е���Ҫ��Ӧ���÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ԫ�����ڱ��е������ڵ�IIA��Ԫ�أ���������������ȷ���ǣ� ��
A. �صĽ����Աȸ���B. ��������ˮ��Ӧ����H2
C. �ڻ������г�+2��D. ̼����������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڱ���һ�����ŵ���Ԫ�ش�������Ԫ�ش�����δ������������Ԫ�ش�����Ϊ119��Ԫ�ذ��ź����ķ���( )
A. �ڰ����ڵ�IA�� B. �������ڵڢ�A��
C. �������ڵ�0�� D. �������ڢ�A��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С�齫V1mL0.50mol/LH2SO4��Һ��V2mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ��װ�ú�ʵ��������ͼ��ʾ(ʵ����ʼ�ձ���V1 +V2=50mL)���ش��������⣺

��1����ͼ��ʾʵ��װ������һ�����ԵĴ���________________��
��2��Ϊ�˼���ʵ����ʵ������н�NaOH��Һ________________ (ѡ����һ���������ֶ����������ʢ��ϡ�����С�ձ��С���Һ��Ϻ�ȷ��ȡ�����Һ��____________����Ϊ��ֹ�¶ȡ�
��3���о�С������ʵ��ʱ�����¶�________ 22��C(����������� ��������������������
��4������ɼ�ͼ�ο�֪�˷�Ӧ����NaOH��Һ��Ũ��ӦΪ_________ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�뻷�����������彡�����������ϵ�ϢϢ��ء�
��1����Ȼˮ�����ʽ϶࣮�������������ClO2�����ʴ�����������á�����ClO2 ��������__������������Al3+ˮ������ӷ���ʽ��_________________________��
��2����װ��������ࡣ���������в�������Ȼ��ά����__________������ţ���
a������ b��˿��ë c�����ںͽ���
��3��������ʴ����ɾ�ľ�����ʧ�������ڳ�ʪ�Ŀ����и�����ʴ����Ҫԭ���Ǹ����������̼��������С��___________�������绯ѧ��ʴ��
��4���������������������ҹ��������̽�����Ⱥӣ����Ʊ�������ʹ����þ���Ͻ�þ���Ͻ���ŵ���_________��a��ǿ�ȴ� b���ܶȴ� c����Ե�Ժ� d����ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ����������DO���Ǻ���ˮ���Ծ�������һ��ָ�꣬ͨ����ÿ��ˮ���ܽ������ӵ�������ʾ����λmg��L-1���ҹ����ر�ˮ�������������涨����������ˮԴ��DO���ܵ���5 mg��L-1��ˮ���ܽ����IJⶨ�������£�
��1����һ����ˮ���м�������MnSO4�ͼ���KI��Һ������MnO��OH��2�������ܷ⾲�ã�
��2����������ϡH2SO4�����裬��MnO��OH��2��I-��ȫ��Ӧ����Mn2+��I2��
��3����Na2S2O3����Һ�ζ����յ㡣
�ⶨ���������ʵ�ת����ϵ���£�
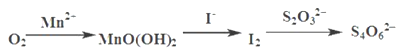
��֪��MnO��OH��2+2I-+4H+ =Mn2++I2+3H2O 2S2O32- +I2= S4O62-+2I-
�ټ���ϡ���������������__________��Na2S2O3����ҺӦװ��______________�ζ�����������ʽ���ʽ����
�ڵζ���������_____________Ϊָʾ�����ﵽ�ζ��յ�ı�־Ϊ____________________��
��д��O2��Mn2+������MnO��OH��2�����ӷ���ʽ____________________��
���������ϡH2SO4��Һ��Ӧ������ҺpH���ͣ��ζ�ʱ��������Ե���д����������һ��ԭ���������ӷ���ʽ��ʾ��__________________��
�����в���ʹ���ˮ������������DO����ʵ��ֵƫ�ߵ���__________________��
A.����1������������ʱû���ܷ� B.װNa2S2O3����Һ�ĵζ���û����ϴ
C.�ζ�ǰ��ƿϴ����û�и��� D.�ζ�ǰ������ȷ���ζ����Ӷ���
��ȡ�ӹ�һ����CaO2��8H2O�ij���ˮ��l00.00mL�������������ⶨˮ���ܽ�����������0.01000
mol��L-1 Na2S2O3����Һ13.50 mL�������ˮ���е��ܽ���Ϊ____________mg��L-1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com