
��1�������£�������ؾ�����Ũ���������һ�����壬������������仯ѧ���������ȷ��ֵġ���ͼ��ʾ����Ũ����С�ĵ���Y�ι�B�ˡ�
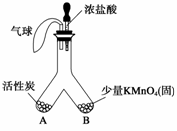
�ٿ�ʼ�۲쵽���������������������������������������������������� ��
��һ��ʱ����ֿɹ۲쵽�������� �������� ���������һ�������������������������������������������������������������������������������� ��
��Ӧ�û���̿�Ĵ����ʿ������������� ���������ﵽ������������Ŀ�ģ���һ�����ɣ���
��2����д��CO2��Na2O2��Ӧ�Ļ�ѧ����ʽ������������������������������ ��
��ijѧ���ж�SO2��Na2O2��Ӧ�����������ƣ�����Ϊ�����жϺ��������������������������� ��
��Ҫ˵�����ɣ��� ����������������������������������������������������������������������������������
�۸�ͬѧ���϶���Ӧ���Ƿ����������ɣ���ʹ����ͼ��ʾװ�ý���ʵ�飨ͼ������̨��װ������ȥ����
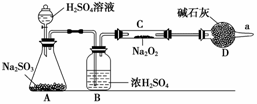
װ����B�������� ����������������������������������������������������������������������������������
D�������� �������������������������������������������������������������������������������������� ��
�����֤����ͬѧ�������ƶϣ� ����������������������������������������������������������������������
������������������������������������������������������������������������������������������������ ��
��1�����л���ɫ��������������������� �ڻ���ɫ��dz���������ȥ ��Ϊ����̿������ܴ�����������ǿ���ɽ�Y�ι��е���������������ʹ�û���ɫ��dz�۷�����ߡ�����̿���֡�����̿С�˰��ȣ��𰸺��������֣�
��2����2CO2+2Na2O2![]() 2Na2CO3+O2
2Na2CO3+O2
�ں��� ���ڹ������ƾ���ǿ�����ԣ��ܽ�+4�۵�������Ϊ+6�۵��������������
��Bװ�����ڸ���SO2���壬��ֹˮ��������Cװ����Na2O2��Ӧ Dװ����Ϊ�˷�ֹ�����е�ˮ��������Cװ����Na2O2��Ӧ����������ͬʱ���չ���SO2���壬���������ļ���ͷ�ֹ��Ⱦ����
�ܼ���Na2SO4:��Ӧ��ȡCװ���еĹ�����������ˮ�����Һ���ȵμ������ữ���ټ���BaCl2��Һ���г�������,��֤����Na2SO4���ɣ�
����O2���ô����ǵ�ϸľ����������ܿ�a����ϸľ�����DZ�����ϸľ����ȼ����֤����O2���ɣ�����������Ҳ�ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���֪pH��2�ĸߵ��ᣨHIO4����Һ��pH��12��NaOH��Һ�������Ϻ�������Һ�����ԣ�0.01mol/L�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH��12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ��
��2��0.01mol/L�ĵ��ᣨHIO3����Һ��pH��12��NaOH��Һ�������Ϻ�������Һ�У�
c(IO3��) c(Na��)������ڡ�����С�ڡ����ڡ�����
��3����֪ij��Һ��ֻ����OH����H�� ��IO4�� ��Na���������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��
A. c(Na��)��c(IO4��)��c(OH��)��c(H��) B. c(IO4��)��c(Na��)��c(OH��)��c(H��)
C. c(Na��)��c(OH��)��c(IO4��)��c(H��) D. c(IO4��)��c(Na��)��c(H��)��c(OH��)
��д���пհף�
������Һ��ֻ��һ�����ʣ���������� ��������������Ũ�ȴ�С˳��Ϊ????????????????
����ѡ��ı�ţ�
����������ϵ��C������ȷ�ģ�����Һ�е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ����ʦ���и����������¿���ѧ�Ծ� ���ͣ������
��8�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��IO��3��Na+�����ʵ����Ĺ�ϵ��n(Na+) n(IO-3)������ڡ�����С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����������¿���ѧ�Ծ� ���ͣ������
��8�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��IO��3��Na+�����ʵ����Ĺ�ϵ��n(Na+) n(IO-3)������ڡ�����С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
��10�֣���������֪pH=2�ĸߵ��ᣨHIO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԣ�0.01mol��L-1�ĵ��ᣨHIO3��������ᣨHMnO4����Һ��pH=12��NaOH��Һ�������ϣ�������Һ�����ԡ���ش��������⣺
��1���ߵ����� ���ǿ�ᡱ�����ᡱ���������� ��
��2��0.01mol��L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ��������������Һ��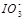 ��Na+��Ũ�ȹ�ϵ��
������ڡ���С�ڡ����ڡ�����
��Na+��Ũ�ȹ�ϵ��
������ڡ���С�ڡ����ڡ�����
��3����֪�ߵ���������̣�MnSO4������Һ�з�Ӧ���ɸ����ᡢ��������ᣬ�˷�Ӧ�Ļ�ԭ���� ���÷�Ӧ�����ӷ���ʽ�ɱ�ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com