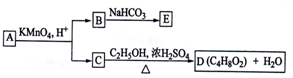
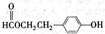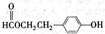ЭМжа AЁЂBЁЂCЁЂDЁЂEОљЮЊгаЛњЛЏКЯЮяЃЎвбжЊЃКCФмИњNaHCO
3ЗЂЩњЗДгІЃЌCКЭDЕФЯрЖдЗжзгжЪСПЯрЕШЃЌЧвEЮЊЮожЇСДЕФЛЏКЯЮяЃЎ
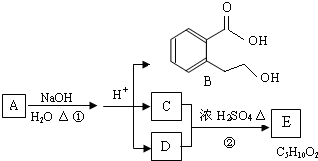
ИљОнЭМЛиД№ЮЪЬтЃК
ЃЈ1ЃЉCЗжзгжаЕФЙйФмЭХУћГЦЪЧЃК
єШЛљ
єШЛљ
ЃЛ
ЯТСаЗДгІжаЃЌЛЏКЯЮяBВЛФмЗЂЩњЕФЗДгІЪЧ
e
e
ЃЈЬюзжФИађКХЃЉЃК
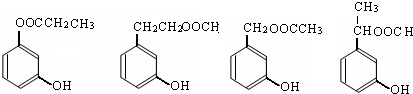
aЁЂМгГЩЗДгІ bЁЂШЁДњЗДгІ cЁЂЯћШЅЗДгІ dЁЂѕЅЛЏЗДгІ eЁЂЫЎНтЗДгІ fЁЂжУЛЛЗДгІ
ЃЈ2ЃЉЗДгІЂкЕФЛЏбЇЗНГЬЪНЪЧ
CH
3COOH+CH
3CH
2CH
2OH
CH
3COOCH
2CH
2CH
3+H
2O
CH
3COOH+CH
3CH
2CH
2OH
CH
3COOCH
2CH
2CH
3+H
2O
ЃЎ
ЃЈ3ЃЉAЕФНсЙЙМђЪНЪЧ
ЃЎ
ЃЈ4ЃЉЭЌЪБЗћКЯЯТСаШ§ИіЬѕМўЕФBЕФЭЌЗжвьЙЙЬхЕФЪ§ФПга
4
4
ИіЃЎ
ЂёЃЎКЌгаМфЖўШЁДњБНЛЗНсЙЙЃЛЂђЃЎЪєгкЗЧЗМЯуЫсѕЅЃЛЂѓЃЎгы FeCl
3 ШмвКЗЂЩњЯдЩЋЗДгІЃЎ
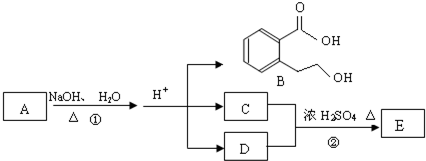
аДГіЦфжаШЮвтвЛИіЭЌЗжвьЙЙЬхЕФНсЙЙМђЪН
аДГіЫФепжЎвЛМДПЩ

аДГіЫФепжЎвЛМДПЩ
ЃЈ5ЃЉГЃЮТЯТЃЌНЋCШмвККЭNaOHШмвКЕШЬхЛ§ЛьКЯЃЌСНжжШмвКЕФХЈЖШКЭЛьКЯКѓЫљЕУШмвКpHШчЯТБэЃК
| ЪЕбщБрКХ |
CЮяжЪЕФСПХЈЖШЃЈmol?L-1ЃЉ |
NaOHЮяжЪЕФСПХЈЖШЃЈmol?L-1ЃЉ |
ЛьКЯШмвКЕФpH |
| m |
0.1 |
0.1 |
pH=9 |
| n |
0.2 |
0.1 |
pHЃМ7 |
ДгmзщЧщПіЗжЮіЃЌЫљЕУЛьКЯШмвКжагЩЫЎЕчРыГіЕФcЃЈOH
-ЃЉ=
10-5
10-5
mol?L
-1ЃЎ
nзщЛьКЯШмвКжаРызгХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ
cЃЈCH3COO-ЃЉЃОcЃЈNa+ЃЉЃОcЃЈH+ЃЉЃОcЃЈOH-ЃЉ
cЃЈCH3COO-ЃЉЃОcЃЈNa+ЃЉЃОcЃЈH+ЃЉЃОcЃЈOH-ЃЉ
ЃЎ











 аДГіЫФепжЎвЛМДПЩ
аДГіЫФепжЎвЛМДПЩ аДГіЫФепжЎвЛМДПЩ
аДГіЫФепжЎвЛМДПЩ