
��ҵ���ú�п����(��FeO��CuO������)���Ƶû���ZnO���������£�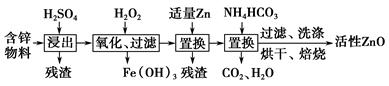
(1)���������У������õ���60%H2SO4(1.5 g��cm��3)����������H2SO4 100 mL��Ҫ18.4 mol��L��1��ŨH2SO4________ mL(����һλС��)��
(2)����������H2O2����Fe(OH)3�������֣�û��Cu(OH)2�������֣�����Һ��c(Fe3��)��2.6��10��18 mol��L��1������Һ��c(Cu2��)��ȡֵ��Χ��________mol��L��1��(��֪Ksp[Fe(OH)3]��2.6��10��39��
Ksp[Cu(OH)2]��2.2��10��20)
(3)����NH4HCO3�����ɵij�������̬��ΪZna(OH)b(CO3)c(a��b��cΪ������)�����ּ�ʽ̼��пA��B�Ļ���A��a��5��b��6�������ɼ�ʽ̼��пA�Ļ�ѧ����ʽΪ__________________________________________________��
(4)ȡϴ�ӡ���ɺ�ļ�ʽ̼��пA��B�Ļ����49.70 g�������ʵ���Ϊ0.10 mol�����±�����ȫ�ֽ�õ�37.26 g ZnO��3.584 L CO2(��״����)��ˮ��ͨ�����������ʽ̼��пB�Ļ�ѧʽ��
(1)49.9(50.0Ҳ����) (2)��2.2��10��6
(3)5ZnSO4��10NH4HCO3=Zn5(OH)6(CO3)2����5(NH4)2SO4��8CO2����2H2O
(4)������0.1 mol�������ȫ�ֽ�õ�ZnO��CO2��H2O�����ʵ����ֱ�Ϊ0.46 mol��0.16 mol��0.3 mol
��֪1 mol�������ƽ����4.6 mol Zn��1.6 mol C��6 mol H����֪1 mol A�к�HΪ6 mol����CΪ2 mol����1 mol B�к�HΪ6 mol����CΪ1 mol������B�Ļ�ѧʽ���Ա�ʾΪZnx(OH)6CO3���ɻ��ϼ۴�����Ϊ��ó�x��4����B�Ļ�ѧʽΪZn4(OH)6CO3(���������ⷨ����)
����


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ijҺ�廯����X2Y4�����������ȼ�ϡ�16g X2Y4��һ������O2��ǡ����ȫȼ�գ���Ӧ����ʽΪX2Y4(l)��O2(g)===X2(g)��2Y2O(l)����ȴ���״���²������������Ϊ11.2 L�����ܶ�Ϊ1.25g/L����
��1����ӦǰO2�����V(O2)Ϊ________��
��2��X2��Ħ������Ϊ________��YԪ�ص�������________��
��3������Ӧ����0.1mol X2����ת�Ƶ��ӵ����ʵ���Ϊ________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ɵ�������NaHCO3��KHCO3��ɵĻ����a g����100 mL���ᷴӦ��(�����漰������������Ա�״���ƣ����ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)
(1)�û������NaHCO3��KHCO3�����ʵ���֮��Ϊ ��
(2)��̼������������ǡ����ȫ��Ӧ����������HCl�����ʵ���Ϊ mol��
(3)����������������CO2�����Ϊ L��
(4)�����Ӧ��̼��������ʣ�࣬���������Ҫ��������CO2�����������Ҫ֪�� ��
(5)��NaHCO3��KHCO3�����Ե�������ϣ���a g����������������������ȫ��Ӧʱ����CO2�����[V(CO2)]��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��ͬ�¡�ͬѹ�£�ʵ����CO��N2��O2��������Ļ��������ܶ���H2��14.5��������O2����������Ϊ ��������CO��N2�����ʵ���֮��Ϊ1��1��������������Ԫ�ص���������Ϊ ��
(2)��ͬ�����£�ijCl2��O2�������100 mLǡ����150 mL H2��������HCl��H2O������������Cl2��O2�������Ϊ ����������ƽ����Է�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijУ��ѧС��ѧ�����С�������Է��������IJⶨ����ʵ�顣�������£����������ݻ�����ȵ���ƿ�ռ����壬�����ռ����������ƿ���������ݼ��±�(�ѻ���ɱ�״���µ���ֵ)��
| ���� | ��ƿ�������������(g) |
| A | 48.4082 |
| B | 48.4082 |
| C | 48.4082 |
| D | 48.4342 |
| E | 48.8762 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ����Ͻ�����������ʹ�õĽ������ϡ�
(1)����ͭ��ȡ�������ַ�ʽ�ѻ�(����)

(2)��1��Cu2O������(�ṹ����ͼ��ʾ)��Cuԭ����λ��Ϊ__________��
(3)��ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�
�ٵ����Ļ�ѧʽ����������ʽ��ʾΪ____________��
�ڵ�����SO42���Ŀռ乹��Ϊ________��H2O��Oԭ�ӵ��ӻ�����Ϊ________��
��ij��ȤС���ȡ2.500 g�������壬������ʹ��ʧˮ����ȷ�ⶨ��ͬ�¶���ʣ�������������õ���ͼ��ʾ��ʵ����ʾ��ͼ������˵����ȷ����(����)
| A������ӳ�������105 ��Ĺ�����ֻ��������� |
| B�������������γ���λ����4��ˮ����ͬʱʧȥ |
| C��120 ��ʱ��ʣ�����Ļ�ѧʽ��CuSO4��H2O |
| D������������ʧˮʱ���˷�����������С��ͬ�������е�ˮ���ӿ��Է�Ϊ3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ĺ�ҵ�Ʊ���һ����Ҫ�Ļ����������̣��������������л����������Ⱦ����Ҫ�����������п��ǵ���ɫ���ա�
Iβ�������պ��ۺ����á�
�Թ�ҵ�������β������ˮ��ʯ��ʯ����̿��̼����狀�KCIΪԭ�Ͽ��Ժϳ��ơ�����ء�������淋����ʡ��ϳ�·�����£�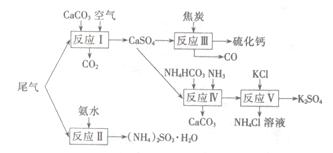
��1����ӦIII���������뻹ԭ�������ʵ���֮��Ϊ ��
��2����Ӧ���Ļ�ѧ����ʽΪ ��
��3����ӦV��25�桢40%���Ҷ�����Һ�н��У��÷�Ӧ��˳�����е�ԭ��Ϊ ��
������Ļ������á�
SO2�Ĵ�������ʹ�õĴ���ΪV2O5��ʵ�������У�������ʹ��һ��ʱ��Ậ��V2O5��VOSO4��SiO2�ȣ�����VOSO4��������ˮ������V2O5������Ҫ�������£�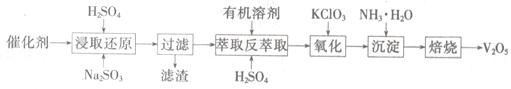
��4��������ȡʹ�õ�������������һ������ʱ������____ ��������
��5����ȡ��ԭ���̵IJ���֮һ��VOSO4����Ӧ�Ļ�ѧ����ʽΪ ��
�������̵Ļ�ѧ����ʽΪKClO3+6VOSO4+3H2SO4= 2(VO)2(SO4)3+KCl+3H2O�������������Լ�Na2SO3��KC1O3�����ʵ���֮��Ϊ12��7����ô�����V2O5��VOSO4�����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Դ����Ľ�һ��ͻ���������Ȼ�ѧѭ��������о��ܵ��������ҵ�������������о����֣�����������������(MnFe2O4)Ҳ���������Ȼ�ѧѭ���ֽ�ˮ���⣬MnFe2O4���Ʊ��������£�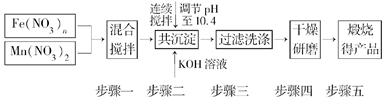
(1)ԭ��Fe(NO3)n��n��________��Ͷ��ԭ��Fe(NO3)n��Mn(NO3)2�����ʵ���֮��ӦΪ________��
(2)������С��������衱��Ŀ����__________________________________________
��������ϴ�Ӹɾ��ı���________________________________________________
(3)����MnFe2O4�Ȼ�ѧѭ������ķ�Ӧ�ɱ�ʾΪ��
MnFe2O4 MnFe2O4��x��O2����
MnFe2O4��x��O2����
MnFe2O4��x��xH2O MnFe2O4��xH2��
MnFe2O4��xH2��
�������������������Ӧ���ش��������⣺
����MnFe2O4��x��x��0.8����MnFe2O4��x��Fe2��ռȫ����Ԫ�صİٷ���Ϊ________��
�ڸ��Ȼ�ѧѭ�����ⷨ���ŵ���_____________________��________________________ (�����㼴��)��
���Ȼ�ѧѭ�����������в���֮������һ���Ľ����о�������___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����ý�̿��ʯ��Ҥ��ȼ�շ��ȣ�ʹʯ��ʯ�ֽ�����CO2����Ҫ��Ӧ���£�
C+O2��CO2 �٣� CaCO3��CO2��+CaO ��
��1����̼���95%��ʯ��ʯ2.0 t������ȫ�ֽ⣨�����ʲ��ֽ⣩���ɵñ�״����CO2�����Ϊ_________________m3��
��2��������CaCO3�ͽ�̿���٢���ȫ��Ӧ����Ҥ�������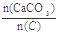 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
��3��ij��Ҥ���ɷ����£�O2 0.2%��CO 0.2%��CO2 41.6%������ΪN2����˴�Ҥ������� Ϊ��ֵ��
Ϊ��ֵ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com