
�����ɵ�������NaHCO3��KHCO3��ɵĻ����a g����100 mL���ᷴӦ��(�����漰������������Ա�״���ƣ����ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)
(1)�û������NaHCO3��KHCO3�����ʵ���֮��Ϊ ��
(2)��̼������������ǡ����ȫ��Ӧ����������HCl�����ʵ���Ϊ mol��
(3)����������������CO2�����Ϊ L��
(4)�����Ӧ��̼��������ʣ�࣬���������Ҫ��������CO2�����������Ҫ֪�� ��
(5)��NaHCO3��KHCO3�����Ե�������ϣ���a g����������������������ȫ��Ӧʱ����CO2�����[V(CO2)]��Χ�� ��
 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��Ũ��������һ�����ʵ���Ũ�ȵ�������Һ�����в��������������ҺŨ��ƫ�ߵ��ǣ� ��
| A���ܽ�����Һδ��ȴ�����¾�ת��������ƿ�� |
| B��������תҡ�Ⱥ�Һ����ڿ̶��ߣ��ټ�����ˮ��Һ����͵�ǡ����̶�����ƽ |
| C��ϴ���ձ��Ͳ���������Һת��������ƿ�� |
| D������ʱ���۾����ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�ʵ��������һ�����ʵ���Ũ�ȵ���Һ���辭���㡢�������ܽ⡢��Һ��
ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�Ȳ��衣��������0.2mol/L��CuSO4��Һ500mL��
�ش��������⣺
��1����������ƽ��ȡCuSO4��5H2O����������� g��
��2�����ܽⲢ��ȴ�����Һת�ƵĹ������õ��IJ��������� ��
��3������ʱ����ˮ����̶���1-2cmʱ���ý�ͷ�ιܵμ�����ˮ�� ��
��4�����������ʹ������ҺŨ��ƫ�͵��� ��������ţ�
A����ʱ����
Bδ��ϴ���ձ������Һת��������ƿ
C������ˮʱ�����������˿̶���
D������մ�����ʣ�����ʹ����������룩
E������ƿʹ��ǰδ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�ֹ��ɽ���Ԫ�أ�ͨ�������Ͻ���ֵ����Ӽ�������Ԫ�ؿ���ǿ�Ͻ��ǿ�ȡ�Ӳ�ȡ��ɺ��Լ����ԣ�������ǿ�����¼���ʴ���ܡ���ͼ�ǻ����������Ʊ����������Ҫ����ͼ��
(1)д����Ӧ�ٵĻ�ѧ����ʽ�� ��
(2)��Ӧ�ٵ�β�����������ã�д��Ӧ�ø�β���Ƶõ�������Ҫ��ѧ�Լ� ��
(3)�����ʵ����ģ�����1�Ͳ���2������Ҫʹ�õ���Ҫ���������� ��
(4)���ڿ��������������������⣬����������������������Һ���������ƣ��������ⲻ���������ϡ���ᡣ�����ƵĻ�ѧʽΪ ��
(5)��ҵ���Ʊ���ԭ������CO��H2�ķ�Ӧԭ��ΪCO2��CH4 2CO��2H2��CH4��H2O
2CO��2H2��CH4��H2O CO��3H2���������������Ϊ80%��a L(��״��)��Ȼ��������������̼��ˮ�����Ļ�����ڸ����·�Ӧ������ת����Ϊ90%���ò����Ļ�ԭ������(CO��H2)��ԭMoO3���⣬�������������������Ϊ ��
CO��3H2���������������Ϊ80%��a L(��״��)��Ȼ��������������̼��ˮ�����Ļ�����ڸ����·�Ӧ������ת����Ϊ90%���ò����Ļ�ԭ������(CO��H2)��ԭMoO3���⣬�������������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��
__________________________________________________(��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ_________________��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ____________________����MnO2Zn������ƣ�K2FeO4ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ_____________________���õ���ܷ�Ӧ�����ڷ���ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������(FeSO4��7H2O)��ҽҩ�Ͽ�����Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�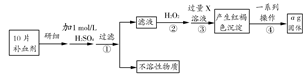
��ش��������⣺
(1)����ڼ������H2O2��Ŀ���� ��
(2)������з�Ӧ�����ӷ���ʽΪ ��
(3)������е�һϵ�в�������Ϊ�����ˡ� �����ա� ��������
(4)��ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ g(�ú�a�Ĵ���ʽ��ʾ�����û���)��
(5)��С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ����Ӧԭ��Ϊ��5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����Ҫ�IJ�������Ϊ ��
������ʵ���е�KMnO4��Һ��Ҫ�ữ���������ữ������ ��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ��ʵ��װ�ÿ������ⶨ������Ԫ�ص�ij������X�ķ���ʽ��
��ע����A��װ��240 mL����X������ͨ������������װ�к��ȵ�����ͭ�IJ�����B��ʹ֮��ȫ��Ӧ���õ�����ʵ������ʵ��ǰB����20.32 g��ʵ���B����20.00 g��B���еĺ�ɫ��ĩ��ɺ�ɫ��ĩ����C�����ռ�������ɫҺ����ˮ����ע����D���ռ����������ǵ������Իش��������⣺
(1)X��������________��________Ԫ����ɵġ�
(2)��240 mL X������ȫ��Ӧ���ռ����ĵ���������0.28 g������ʵ��ʱ�¶Ⱥ�ѹǿ����1 mol X����������24 000 mL����X��Ħ��������________��
(3)ͨ�����㣬ȷ��X�ķ���ʽΪ________��
(4)д��B�з�����Ӧ�Ļ�ѧ����ʽ(X�ڸ������²������ֽⷴӦ)________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���ú�п����(��FeO��CuO������)���Ƶû���ZnO���������£�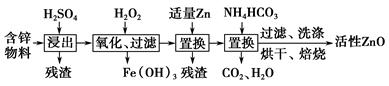
(1)���������У������õ���60%H2SO4(1.5 g��cm��3)����������H2SO4 100 mL��Ҫ18.4 mol��L��1��ŨH2SO4________ mL(����һλС��)��
(2)����������H2O2����Fe(OH)3�������֣�û��Cu(OH)2�������֣�����Һ��c(Fe3��)��2.6��10��18 mol��L��1������Һ��c(Cu2��)��ȡֵ��Χ��________mol��L��1��(��֪Ksp[Fe(OH)3]��2.6��10��39��
Ksp[Cu(OH)2]��2.2��10��20)
(3)����NH4HCO3�����ɵij�������̬��ΪZna(OH)b(CO3)c(a��b��cΪ������)�����ּ�ʽ̼��пA��B�Ļ���A��a��5��b��6�������ɼ�ʽ̼��пA�Ļ�ѧ����ʽΪ__________________________________________________��
(4)ȡϴ�ӡ���ɺ�ļ�ʽ̼��пA��B�Ļ����49.70 g�������ʵ���Ϊ0.10 mol�����±�����ȫ�ֽ�õ�37.26 g ZnO��3.584 L CO2(��״����)��ˮ��ͨ�����������ʽ̼��пB�Ļ�ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2��SO4��3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�
��1�������ա������з�Ӧ�Ļ�ѧ����ʽΪ Al2��SO4��3��
Al2��SO4��3�� S
S
 Al2O3��
Al2O3�� ______����
______����
��2��������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________________________________________��
��3��������pH������ˡ�ϴ��Al��OH��3������֤��������ϴ�Ӹɾ���ʵ�������������________��
��4����ĸҺ���пɻ��յ�������________��
��5���������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com