
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2��SO4��3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�
��1�������ա������з�Ӧ�Ļ�ѧ����ʽΪ Al2��SO4��3��
Al2��SO4��3�� S
S
 Al2O3��
Al2O3�� ______����
______����
��2��������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________________________________________��
��3��������pH������ˡ�ϴ��Al��OH��3������֤��������ϴ�Ӹɾ���ʵ�������������________��
��4����ĸҺ���пɻ��յ�������________��
��5���������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ɵ�������NaHCO3��KHCO3��ɵĻ����a g����100 mL���ᷴӦ��(�����漰������������Ա�״���ƣ����ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)
(1)�û������NaHCO3��KHCO3�����ʵ���֮��Ϊ ��
(2)��̼������������ǡ����ȫ��Ӧ����������HCl�����ʵ���Ϊ mol��
(3)����������������CO2�����Ϊ L��
(4)�����Ӧ��̼��������ʣ�࣬���������Ҫ��������CO2�����������Ҫ֪�� ��
(5)��NaHCO3��KHCO3�����Ե�������ϣ���a g����������������������ȫ��Ӧʱ����CO2�����[V(CO2)]��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ĺ�ҵ�Ʊ���һ����Ҫ�Ļ����������̣��������������л����������Ⱦ����Ҫ�����������п��ǵ���ɫ���ա�
Iβ�������պ��ۺ����á�
�Թ�ҵ�������β������ˮ��ʯ��ʯ����̿��̼����狀�KCIΪԭ�Ͽ��Ժϳ��ơ�����ء�������淋����ʡ��ϳ�·�����£�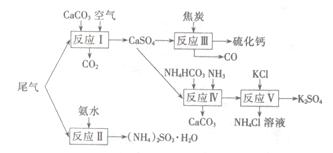
��1����ӦIII���������뻹ԭ�������ʵ���֮��Ϊ ��
��2����Ӧ���Ļ�ѧ����ʽΪ ��
��3����ӦV��25�桢40%���Ҷ�����Һ�н��У��÷�Ӧ��˳�����е�ԭ��Ϊ ��
������Ļ������á�
SO2�Ĵ�������ʹ�õĴ���ΪV2O5��ʵ�������У�������ʹ��һ��ʱ��Ậ��V2O5��VOSO4��SiO2�ȣ�����VOSO4��������ˮ������V2O5������Ҫ�������£�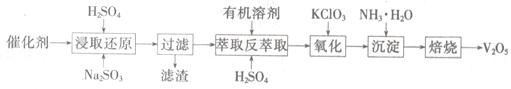
��4��������ȡʹ�õ�������������һ������ʱ������____ ��������
��5����ȡ��ԭ���̵IJ���֮һ��VOSO4����Ӧ�Ļ�ѧ����ʽΪ ��
�������̵Ļ�ѧ����ʽΪKClO3+6VOSO4+3H2SO4= 2(VO)2(SO4)3+KCl+3H2O�������������Լ�Na2SO3��KC1O3�����ʵ���֮��Ϊ12��7����ô�����V2O5��VOSO4�����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Դ����Ľ�һ��ͻ���������Ȼ�ѧѭ��������о��ܵ��������ҵ�������������о����֣�����������������(MnFe2O4)Ҳ���������Ȼ�ѧѭ���ֽ�ˮ���⣬MnFe2O4���Ʊ��������£�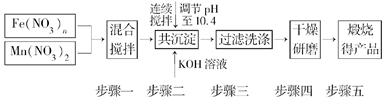
(1)ԭ��Fe(NO3)n��n��________��Ͷ��ԭ��Fe(NO3)n��Mn(NO3)2�����ʵ���֮��ӦΪ________��
(2)������С��������衱��Ŀ����__________________________________________
��������ϴ�Ӹɾ��ı���________________________________________________
(3)����MnFe2O4�Ȼ�ѧѭ������ķ�Ӧ�ɱ�ʾΪ��
MnFe2O4 MnFe2O4��x��O2����
MnFe2O4��x��O2����
MnFe2O4��x��xH2O MnFe2O4��xH2��
MnFe2O4��xH2��
�������������������Ӧ���ش��������⣺
����MnFe2O4��x��x��0.8����MnFe2O4��x��Fe2��ռȫ����Ԫ�صİٷ���Ϊ________��
�ڸ��Ȼ�ѧѭ�����ⷨ���ŵ���_____________________��________________________ (�����㼴��)��
���Ȼ�ѧѭ�����������в���֮������һ���Ľ����о�������___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ������һƿ�����ơ�84����Һ��������������Ϻ�����Һ��װ˵���õ�������Ϣ��
��84����Һ������25%NaClO 1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á�
�����������Ϣ�����֪ʶ�ش��������⣺
(1)�á�84����Һ�������ʵ���Ũ��Ϊ________ mol��L��1��
(2)��ͬѧȡ100 mL�á�84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na��)��________ mol��L��1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm��3)��
(3)ijʵ������480 mL��25%NaClO������Һ����ͬѧ���ĸá�84����Һ�����䷽������NaClO�������Ƹ�����Һ��
������˵����ȷ����________��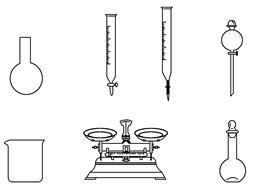
A������ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D����Ҫ������NaClO��������Ϊ143 g
�������ƹ����У����в�������ʹ���Ƶ���Һ��Ũ��ƫ�����________��
A���ձ�����Һת�Ƶ�����ƿ��ʱ��δϴ���ձ�
B������ʱ�����ӿ̶���
C������ʱ�����ӿ̶���
D����Һʱ��������Һ�彦��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.ʵ��������1mol/L Na2CO3��Һ250ml��
��1����Ҫ����Na2CO3 g����2������Һ�е���������ĿΪ ����
��3����Ҫ���ʵ���Ũ��Ϊ5mol/L ��Na2CO3��Һ ml��
��4��������Һ������ϡ���ᷴӦ�������������ڱ�״���µ����Ϊ L��
��5�����Ƹ���Һ�IJ���˳����(����ĸ��ʾ,���ظ�ʹ��) ��
| A������ | B��ϴ�� | C������ | D���ܽ� E��ҡ�� F��ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�̷���FeSO4��7H2O���ڻ�ѧ�ϳ���������ԭ������������ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷���
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ ����>��<��=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO3 20�ˣ�����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL2mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS������������������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����ý�̿��ʯ��Ҥ��ȼ�շ��ȣ�ʹʯ��ʯ�ֽ�����CO2����Ҫ��Ӧ���£�
C+O2��CO2 �٣� CaCO3��CO2��+CaO ��
��1����̼���95%��ʯ��ʯ2.0 t������ȫ�ֽ⣨�����ʲ��ֽ⣩���ɵñ�״����CO2�����Ϊ_________________m3��
��2��������CaCO3�ͽ�̿���٢���ȫ��Ӧ����Ҥ�������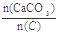 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
��3��ij��Ҥ���ɷ����£�O2 0.2%��CO 0.2%��CO2 41.6%������ΪN2����˴�Ҥ������� Ϊ��ֵ��
Ϊ��ֵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����Ҫ0.10mol/LNaOH��Һ470mL��������Һ����������ش��������⣺
��1��ʵ���г���������ƽ���ձ�������������Ͳ��ҩ�����Ҫ�����������У� ��
��2�����ݼ����֪������NaOH������Ϊ g��
��3������ʱ��������ƿ����Һ�İ�Һ��������̶������У��Ǻ�ƿ�������һ�������� ��
��4������ʱ,�������ˮ�����̶��ߣ������ȡ�Ĵ�ʩ�ǣ� ��
��5�����в���������Ũ���к�Ӱ�죨��д��ĸ��
ƫ�͵��� ����Ӱ����� ��
| A������������������룻 |
| B����NaOH����ֽ���ϳ����� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У� |
| D������ʱ���ӿ̶��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com