
�̷���FeSO4��7H2O���ڻ�ѧ�ϳ���������ԭ������������ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷���
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ ����>��<��=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO3 20�ˣ�����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL2mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS������������������λС������
��1��7.14 mol��L��1 ; > ��2��+2�֣�
��2��0.77��2�֣�
��3��FeSO4��Fe2(SO4)3��10H2O��2�֣�
��4����42��60mL��3�֣��� ��0.33��3�֣�
���������������1����Ũ��������ΪxmL��1.4x��50%/(98*x*0.001)=7.14 mol��L��1����50%��������30%��������ܶȷֱ�ΪA,B�����õ���Һ����������Ϊ(0.5A+0.3B)/(A+B)����ΪA>B���Ը�ʽ����40%��
��2��������������98%Ũ�����м���ͨ��SO3 ,��Ϊ20%ָ��������,20%�ķ�������ɱ�ʾΪH2O��nSO3���Ʊ���Դ������һ��������ΪH2SO4����n-1��SO3���ɸû������H2SO4���ܼ�����SO3�����ʣ���������ϵ�ɵ� 98 ��80��n-1���� ="(1-20%):" 20%�����n="1.3" ���������������б仯�� H2O��1.3SO3��Ϊ1/1.3H2O��SO3����n=0.77��
��3�������������ó��������ᱵ��9.32�˵����ʵ���Ϊ0.04mol,��n(SO42-)=0.04mol��112mL����״�������������ʵ���Ϊ0.005mol,����Cl2---2Fe2+��ϵʽ�����n(Fe2+)=0.01mol��˵��ԭ�����д���FeSO40.01mol������Ϊ1.52g��n(Fe2(SO4)3)=��0.04-0.01��/3=0.01mol������Ϊ4g,��ᾧˮ�����ʵ���Ϊ��7.32-1.52-4��/18=0.1mol�����ߵ����ʵ���֮��Ϊ1��1��10���ʾ���Ļ�ѧʽΪFeSO4��Fe2(SO4)3��10H2O��
��4����200mL2mol/Lϡ������Һ�����ʵ���Ϊ��0.20L��2mol/L=0.4mol�������������ȫ��ΪCu2S����Ҫ����������ʵ���Ϊx��
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
3 16
8.64/160=0.054 x
x=0.288mol,��ʣ����������ʵ�����Ӧ��ʣ�����������ʵ���Ϊ0.4mol-0.228mol=0.112mol��0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O������4H+----3Fe2����ϵʽ�����n(Fe2��)=0.084mol������Ҫ 2 mol/L (NH4)2Fe(SO4)2��Һ�����V =42ml������ͬ�������ټ����������ȫ��ΪCuS����Ҫ��������ʵ���Ϊx��
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
8 3
x 8.64/96=0.09
x=0.24mol,ʣ�����������ʵ���Ϊ0.4mol-0.24mol=0.16mol��0.16mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O������4H+----3Fe2����ϵʽ�����n(Fe2��)=0.12mol������Ҫ 2 mol/L (NH4)2Fe(SO4)2��Һ�����V =60ml���ʴ�Ϊ��42��V��60��
����V=48����48mL��NH4��2Fe��SO4��2������Һn(Fe2��)=0.096mol����ȫ��Ӧ��Ҫ��������ʵ���Ϊ��
NO3-��3Fe2����4H���� NO����3Fe3+��2H2O 1mol 5mol
0.096 0.128
������������ʵ���Ϊ0.128mol������������ﷴӦ����������ʵ���Ϊ0.4mol-0.128mol=0.272mol����Cu2S�����ʵ���Ϊx��CuS�����ʵ���Ϊy��160x+96y=8.64g.��
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
x 16x/3
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
y 8y/3
16x/3+8y/3=0.272mol ,���y=0.03
��������CuS������2.88g,������������=2.88/8.64=0.33�𣺻������CuS����������Ϊ33%��
���㣺������Ҫ������ݷ���ʽ�����йؼ��㣬�ѶȽϴ�ע����������ʱ���ü��������ݼ����������ȡֵ��Χ��


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ��ʵ��װ�ÿ������ⶨ������Ԫ�ص�ij������X�ķ���ʽ��
��ע����A��װ��240 mL����X������ͨ������������װ�к��ȵ�����ͭ�IJ�����B��ʹ֮��ȫ��Ӧ���õ�����ʵ������ʵ��ǰB����20.32 g��ʵ���B����20.00 g��B���еĺ�ɫ��ĩ��ɺ�ɫ��ĩ����C�����ռ�������ɫҺ����ˮ����ע����D���ռ����������ǵ������Իش��������⣺
(1)X��������________��________Ԫ����ɵġ�
(2)��240 mL X������ȫ��Ӧ���ռ����ĵ���������0.28 g������ʵ��ʱ�¶Ⱥ�ѹǿ����1 mol X����������24 000 mL����X��Ħ��������________��
(3)ͨ�����㣬ȷ��X�ķ���ʽΪ________��
(4)д��B�з�����Ӧ�Ļ�ѧ����ʽ(X�ڸ������²������ֽⷴӦ)________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ⱦ�г����к���������Ŀ�����Ⱦ�⣬�ؽ�����������Һ�������ˮ����ȾҲ�൱���أ���������������ӵ�����ʽϿ��������ƣ�����β������Ҫ�к��ɷ�һ����̼�͵�����������˳��п�����Ⱦ���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ������о��ؽ�������ˮ��Ⱦ�Ĵ������зdz���Ҫ�����壮
��1��һ�������£�NO2��SO2��Ӧ����SO3��NO�������壮�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ�������������Ӧƽ��ʱNO2��SO2�����Ϊ1��6����ƽ�ⳣ��K�� ��
��2����ҵ�ϳ���Na2CO3��Һ���շ����������������NO��NO2�Ļ����Ϊ������
��֪��NO������Na2CO3��Һ��Ӧ��
NO��NO2��Na2CO3��2NaNO2��CO2��2NO2��Na2CO3��NaNO2��NaNO3��CO2
��������Na2CO3��Һ��ȫ����NO��NO2�Ļ���ÿ����22.4L����״����CO2��ȫ���ݳ���ʱ������Һ����������44g������������NO��NO2�������Ϊ ��
��3����ͼ��MCFCȼ�ϵ�أ�������ˮú����CO��H2��Ϊȼ�ϣ�һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʣ�AΪ��ص� ����ѡ�������������д��B���缫��Ӧʽ ��
 ��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
��֪��
�ں���6�۸��ķ�ˮ�м���һ���������������������ʹ��6�۸���ԭ�ɣ�3�۸����ٵ�����ҺpH��6��8֮�䣬ʹFe3����Cr3��ת��ΪFe(OH)3��Cr(OH)3��������ȥ�������ӷ���ʽ��ʾ��ҺpH���ܳ���10��ԭ�� ��
��5������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У�
CrO3����ǿ�����ԣ����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯��ͼ��ʾ����B��ʱʣ�����ijɷ��� ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2��SO4��3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�
��1�������ա������з�Ӧ�Ļ�ѧ����ʽΪ Al2��SO4��3��
Al2��SO4��3�� S
S
 Al2O3��
Al2O3�� ______����
______����
��2��������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________________________________________��
��3��������pH������ˡ�ϴ��Al��OH��3������֤��������ϴ�Ӹɾ���ʵ�������������________��
��4����ĸҺ���пɻ��յ�������________��
��5���������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��200 mL ij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ�õ�Na2CO3��NaHCO3�Ļ����Һ��������������Һ�У���εμ�2 mol��L��1�����ᣬ����������������������������ϵ��ͼ��ʾ��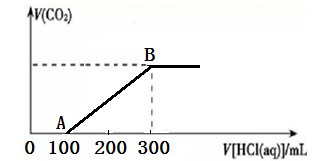
��1��OA�Ρ�AB�η�����Ӧ�����ӷ���ʽ__________________��__________________��
��2��B��ʱ����Ӧ������Һ�����ʵ����ʵ���Ũ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����98%��ŨH2SO4�� =1��84g/cm3�����Ƴ�Ũ��Ϊ0��5mol/L��ϡ����500mL��
=1��84g/cm3�����Ƴ�Ũ��Ϊ0��5mol/L��ϡ����500mL��
��1���뽫���в�������ȷ��������ں����ϣ�
| A������Ͳ��ȡŨH2SO4 |
| B�������ߵ�ҡ�� |
| C���ý�ͷ�ιܼ�ˮ���̶� |
| D��ϴ���ձ��ڱںͲ�����������ϴҺת������ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Իش��������⣺
��1����֪24��A��40��Bǡ����ȫ��Ӧ����0��8molC��32��D����C��Ħ������Ϊ ��
��2����ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ mol/L��
����ʵ��������450mL2��38 mol/L��ϡ���ᣬ���ø�Ũ����________ mL,����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������______________________(����������)��
��ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________2��38mol/L (����ڡ������ڡ���С�ڡ�����ͬ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ������Һʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ��ǩ��������۲��ǩ���������ݺ���㣺
��1������Һ�����ʵ���Ũ��Ϊ mol��L��1���������2λ��Ч���֣���
��2������Һ���ܶ�Ϊ g��mL��1��
��3������Ӹ�ƿ��ȡ��75g������ע��Һ�������Ϊ15%��ע��Һ����Ҫ���� g�����ǹ��壨��ȷ��0.1g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)ͬ��ͬѹ�£�ͬ�����NH3��H2S�������������___________��ͬ������NH3��H2S������������__________��ͬ������NH3��H2S������������ԭ�Ӹ�������___________��������������ԭ�Ӹ�����ȣ����ǵ����ʵ�������________��
(2)Na2SO4??10H2O��Ħ��������__________��483gNa2SO4??10H2O������Na2SO4??10H2O�����ʵ�����_______������Na�������ʵ�����_________������H2O���ӵ���Ŀ��_______����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com