
������������(FeSO4��7H2O)��ҽҩ�Ͽ�����Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�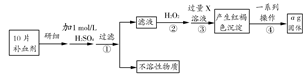
��ش��������⣺
(1)����ڼ������H2O2��Ŀ���� ��
(2)������з�Ӧ�����ӷ���ʽΪ ��
(3)������е�һϵ�в�������Ϊ�����ˡ� �����ա� ��������
(4)��ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ g(�ú�a�Ĵ���ʽ��ʾ�����û���)��
(5)��С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ����Ӧԭ��Ϊ��5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����Ҫ�IJ�������Ϊ ��
������ʵ���е�KMnO4��Һ��Ҫ�ữ���������ữ������ ��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ӵ��ε���Ҫ�ɷ���NaCl����������������KIO3������˵������ȷ����
| A��KIO3��NaClΪ���ӻ������ֻ�����Ӽ� |
B��KIO3�������ӵĽṹʾ��ͼΪ |
| C��23Na37Cl����������������֮����8��7 |
| D���ε���ĽṹʽΪH��O��I |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪һ������������������ȼ�գ����û������100 mL 3��00 mol/L��NaOH��Һ���ܶ�Ϊ1��12 g/mL��ǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0��0500 mol��
��1��ԭNaOH��Һ����������Ϊ ��
��2��������Һ��Cl�������ʵ���Ϊ mol��
��3�����������Ͳμӷ�Ӧ�����������ʵ���֮��n(Cl2)��n(H2)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ᡢ��ˮ���ܶ�������ˮ���Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
| | ���ʵ����ʵ���Ũ��/mol��L��1 | ��Һ���ܶ�/g��cm��3 |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
 c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________
c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________ c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��
c2 mol��L��1(���Ϻ���Һ������仯���Բ���)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ɵ�������NaHCO3��KHCO3��ɵĻ����a g����100 mL���ᷴӦ��(�����漰������������Ա�״���ƣ����ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)
(1)�û������NaHCO3��KHCO3�����ʵ���֮��Ϊ ��
(2)��̼������������ǡ����ȫ��Ӧ����������HCl�����ʵ���Ϊ mol��
(3)����������������CO2�����Ϊ L��
(4)�����Ӧ��̼��������ʣ�࣬���������Ҫ��������CO2�����������Ҫ֪�� ��
(5)��NaHCO3��KHCO3�����Ե�������ϣ���a g����������������������ȫ��Ӧʱ����CO2�����[V(CO2)]��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������и��������������ݣ��ɷֱ�����䡰���ʵ����������������ʵ����ʵ���
Ũ�ȡ������жϲ���⡣
(1)��NA��ʾ�����ӵ���������ֵ����ij����������ҺV L�к���N��OH��������������Һ��______________________Ϊ__________________��
(2)��֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ��________Ϊ________��
(3)��֪��״����1���ˮ���ܽ�500������Ȼ��⣬��������״�����Ȼ��ⱥ����Һ��__________Ϊ________��
(4)��֪��100 mL�Ȼ�����ˮ��Һ�����������գ��ɵõ���ɫ����b g��������ԭ�Ȼ�����Һ��________Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��ͬ�¡�ͬѹ�£�ʵ����CO��N2��O2��������Ļ��������ܶ���H2��14.5��������O2����������Ϊ ��������CO��N2�����ʵ���֮��Ϊ1��1��������������Ԫ�ص���������Ϊ ��
(2)��ͬ�����£�ijCl2��O2�������100 mLǡ����150 mL H2��������HCl��H2O������������Cl2��O2�������Ϊ ����������ƽ����Է�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ����Ͻ�����������ʹ�õĽ������ϡ�
(1)����ͭ��ȡ�������ַ�ʽ�ѻ�(����)

(2)��1��Cu2O������(�ṹ����ͼ��ʾ)��Cuԭ����λ��Ϊ__________��
(3)��ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�
�ٵ����Ļ�ѧʽ����������ʽ��ʾΪ____________��
�ڵ�����SO42���Ŀռ乹��Ϊ________��H2O��Oԭ�ӵ��ӻ�����Ϊ________��
��ij��ȤС���ȡ2.500 g�������壬������ʹ��ʧˮ����ȷ�ⶨ��ͬ�¶���ʣ�������������õ���ͼ��ʾ��ʵ����ʾ��ͼ������˵����ȷ����(����)
| A������ӳ�������105 ��Ĺ�����ֻ��������� |
| B�������������γ���λ����4��ˮ����ͬʱʧȥ |
| C��120 ��ʱ��ʣ�����Ļ�ѧʽ��CuSO4��H2O |
| D������������ʧˮʱ���˷�����������С��ͬ�������е�ˮ���ӿ��Է�Ϊ3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.ʵ��������1mol/L Na2CO3��Һ250ml��
��1����Ҫ����Na2CO3 g����2������Һ�е���������ĿΪ ����
��3����Ҫ���ʵ���Ũ��Ϊ5mol/L ��Na2CO3��Һ ml��
��4��������Һ������ϡ���ᷴӦ�������������ڱ�״���µ����Ϊ L��
��5�����Ƹ���Һ�IJ���˳����(����ĸ��ʾ,���ظ�ʹ��) ��
| A������ | B��ϴ�� | C������ | D���ܽ� E��ҡ�� F��ת�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com