
����Ŀ����3�������Ϊ1 L�ĺ����ܱ������з�����Ӧ��SO2(g)��2NO(g)![]() 2NO2(g)��S(s)���ı�����I�ķ�Ӧ�¶ȣ�ƽ��ʱc( NO2)���¶ȵĹ�ϵ����ͼ��ʾ������˵����ȷ����
2NO2(g)��S(s)���ı�����I�ķ�Ӧ�¶ȣ�ƽ��ʱc( NO2)���¶ȵĹ�ϵ����ͼ��ʾ������˵����ȷ����

���� ��� | �¶�/K | ��ʼ���ʵ���/mol | |||
SO2 | NO | NO2 | S | ||
�� | 0.5 | 0.6 | 0 | 0 | |
�� | T1 | 0.5 | 1 | 0.5 | 1 |
�� | T2 | 0.5 | 0.2 | 1 | 1 |
A. �÷�Ӧ�Ħ�H<0
B. T1ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ![]()
C. �����������������T1ʱ�ﵽƽ�⣬��ѹǿ֮��С��1:2
D. ��T2<T1���ﵽƽ��ʱ����������NO���������С��40%
���𰸡�AD
��������A������ͼ���¶����ߣ�ƽ��ʱNO2Ũ�Ƚ��ͣ�˵���¶����߿�ʹ��ѧƽ������
B��T1�¶ȷ�Ӧ�ﵽƽ��ʱc��NO2��=0.2mol/L�����ݷ�Ӧ����ʽ���㣻
C������pV=nRT�����������ݻ��ͷ�Ӧ�¶�һ������ϵ��ѹǿ����ϵ�л������������ʵ��������ȣ�
D���¶Ƚ��ͣ������ڻ�ѧ��Ӧ������У����ݷ�Ӧ����ʽ�͵�Чƽ���֪ʶ������
A������ͼ���¶����ߣ�ƽ��ʱNO2Ũ�Ƚ��ͣ�˵���¶����߿�ʹ��ѧƽ�������������ӦΪ���ȷ�Ӧ������H��0��A��ȷ��
B��T1�¶ȷ�Ӧ�ﵽƽ��ʱc��NO2��=0.2mol/L����ƽ��ʱc��SO2��=0.5mol/L-0.1mol/L=0.4mol/L��c��NO��=0.6mol/L-0.2mol/L=0.4mol/L�����Է�Ӧ�Ļ�ѧƽ�ⳣ��ΪK=c2(NO2)/c2(NO)c(SO2)=
0.22/0.42��0.4=5/8��B����
C��������������״̬����pV=nRT�����������ݻ��ͷ�Ӧ�¶�һ������ϵ��ѹǿ����ϵ�л������������ʵ��������ȣ��������൱�ڰ�0.75molSO2��1.5molNO��0.75molS��ʼ������S�ǹ��壬���ı�Ũ���̣����������з�Ӧ�ﵽƽ��ʱ������ymolSO2����ƽ��ʱ������ѹ����ΪpI/pII=(0.4+0.4+0.2+0.1)/(2y)��1/(2y)��1/2��C����
D��T2��T1�����¶Ƚ��������ڻ�ѧ��Ӧ������У��������൱����1molSO2��1.2molNO��0.5molS��ʼ��S���Ի�ѧ��Ӧ��ƽ�����Ӱ�죬Ҳ���൱�ڶ��������ѹ����ƽ�ⲻ�����ƶ�����ƽ��ʱNO���������Ϊ40%����������Ļ�ѧ��Ӧ������г̶ȱ�����I������ﵽƽ��ʱ����������NO���������С��40%��D��ȷ����ѡAD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���Ũ�Ⱦ�Ϊ0.1 mol��L-l������4����Һ��
��NaCN��Һ ��NaOH��Һ ��CH3COONa��Һ ��NaHCO3��Һ

(1)��4����ҺpH�ɴ�С��˳����____������ţ������Т���ˮ�����H+Ũ��Ϊ____��
(2)���и�����Ũ���ɴ�С��˳���� ___��
(3)�ܵ�ˮ��ƽ�ⳣ��Kh=___mol/L��
(4)���������Ģۺ͢��еμ������������ԣ�����������������___ �ܣ��>������<"����=����
(5)25��ʱ�����HCN��NaCN�Ļ����Һ��pH=11����![]() ԼΪ____����NaCN��Һ��ͨ������CO2��������Ӧ�����ӷ���ʽΪ��____��
ԼΪ____����NaCN��Һ��ͨ������CO2��������Ӧ�����ӷ���ʽΪ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������Ӧ�ù㷺����ѧʹ�ö����彡�����������������ش�
��1�����������һ������SO2 ���Է�ֹ��Һ��������Ӧ����SO2 ��___�ԡ�
��2��ijˮ������Ԫ����Ҫ��S2O32-��ʽ���ڣ������������£������ӻᵼ��ˮ�����л�ɫ���Dz������д̼�����ζ������ԭ����___________________________________���������ӷ���ʽ˵����
��3��ʵ���Ҳ��õζ����ⶨijˮ�����������κ�����
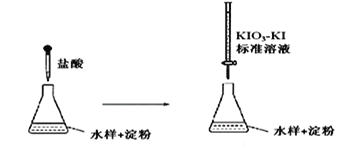
�ζ�ʱ��KIO3 ��KI ����������������I2��5I��+ IO3�� + 6H+ =3I2+3H2O
���ɵ�I2 �ٺ�ˮ���е��������η�Ӧ��I2 + SO32�� + H2O = 2H++2I��+ SO42��
�ٵζ����յ�ʱ��������:________________________________
�����ζ�ǰʢ��Һ�ĵζ���û���ñ�Һ��ϴ����ⶨ�����_________���ƫ��ƫС�����䡱����
�۵ζ��յ�ʱ��100mL��ˮ��������x mL����Һ��������1mL����Һ�൱��SO32��������1g�����ˮ����SO32���ĺ���Ϊ__________g / L
��4����֪�ǽ���������S���ǵ���ɫ�����ĩ��������ˮ��Ϊ����֤��Ԫ�صķǽ����Ա���Ԫ�صķǽ�����ǿ��ij��ѧʵ��С�����������ʵ�飬��ش��������⣺
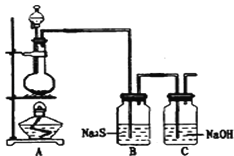
����װ��A��Բ����ƿ��ʢװ�������̣����Һ©����ʢװ���Լ���_____________________
��װ��B��ʵ������Ϊ___________________________��֤����Ԫ�صķǽ����Ա���Ԫ�صķǽ�����ǿ��
��װ��C�з�Ӧ�������ǣ�____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʪ����п�ĵ��Һ��ͬʱ����Cu��CuSO4��������CuCl������ȥCl�������ͶԵ���Ӱ�죬��Ӧԭ�����£�
Cu(s)+Cu2+(aq)![]() 2Cu+(aq) ��H1��a kJ��mol-1
2Cu+(aq) ��H1��a kJ��mol-1
Cl��(aq)+Cu+(aq)![]() CuCl(s) ��H2��b kJ��mol-1
CuCl(s) ��H2��b kJ��mol-1
ʵ���õ��ҺpH����Һ�в���c(Cl��)��Ӱ����ͼ��ʾ������˵����ȷ����

A. ��ҺpHԽ����Ksp(CuCl)����
B. ����Һ�м���ϡ���ᣬ������Cl-��ȥ��
C. ��Ӧ�ﵽƽ������c(Cu2+)��c(Cl��)��С
D. ![]() Cu(s)+
Cu(s)+![]() Cu2+(aq)+Cl��(aq)
Cu2+(aq)+Cl��(aq)![]() CuCl(s)����H��(a+2b) kJ��mol-1
CuCl(s)����H��(a+2b) kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ����������ԭ��Ӧ���ǣ�
A. NH3 + HCl = NH4Cl B. CuO + H2![]() Cu + H2O
Cu + H2O
C. CaCO3 ![]() CaO + CO2�� D. H2SO4 + 2NaOH = Na2SO4 + 2H2O
CaO + CO2�� D. H2SO4 + 2NaOH = Na2SO4 + 2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС���������������ˮ������Ӧ��ʵ���õ�һ�ֺ�ɫ��ĩ������Ϊ̽���ú�ɫ��ĩ���Ƿ���δ��Ӧ����������ֽ���������ʵ�飬װ����ͼ1��ʾ����ش��������⣺
(1)����ˮ������Ӧ�Ļ�ѧ����ʽ��_____________________��
(2)��ͼ1���Ӻ��������װ�õ������ԵIJ���������_______________��
(3)����b��������_____��������b�м�����Լ�������______(��һ��)��
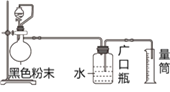
(4)ʵ���й۲쵽��ƿ�������ݲ��������ɫ��ĩ��_______(����������������)���ۣ��������ݵ����ӷ���ʽ��________________________��
(5)�����ɫ��ĩ������Ϊw g����ʱʵ�������²�����������ܶ�Ϊ��g/cm3 ��������������Ͳ��Һ��ƽ�����Ϊa mL�����ɫ��ĩ���������������������Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1��2-����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣
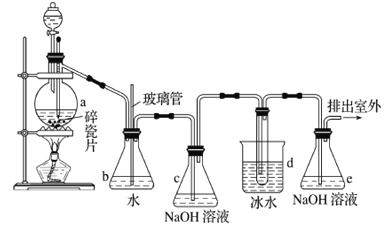
��֪��CH3CH2OH![]() CH2=CH2��+H2O��2CH3CH2OH
CH2=CH2��+H2O��2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
��1��ʵ����ӦѸ�ٽ��¶����ߵ�170�����ҵ�ԭ����______________________________��
��2����ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ�����������д����������ʱƿb�е�����_______________________________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ����_______________________________________________����ȫƿb��������������__________________��
��3������c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������________________________________��
��4����ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ ������ĸ����
A���ؽᾧ B������ C������ D����ȡ
��5��ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У����ʱ��ˮ����������ȴ1��2-��������������⣬��������������____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(SO2Cl2)�����Ȼ������Ȼǻ�������������ҩƷ��Ⱦ�ϡ�������Լ��ȡ��й����ʵIJ����������±���
���� | �۵�/�� | �е�/�� | �������� |
SO2Cl2 | ��54.1 | 69.1 | ������ˮ��Ӧ�������������� ���ֽ⣺SO2Cl2 |
H2SO4 | 10.4 | 338 | ��ˮ���Ҳ��ֽ� |
ʵ�����ø���������Ķ�������������ϳ������ȣ�װ����ͼ��ʾ(�г�������ʡ��)����ش��й����⣺
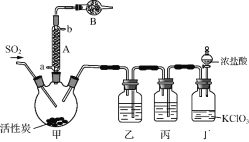
(1) ����A��ȴˮ�Ľ���_______(����a������b��)��
(2) ����B��ʢ�ŵ�ҩƷ��_______��
(3) ʵ������������������������������Ʊ����÷�Ӧ�����ӷ���ʽΪ_______����������������йص�˵������ȷ����_______��
A.��ΪSO2����Ư���ԣ���������ʹƷ����Һ����ˮ������KMnO4��Һ��ʯ����Һ��ɫ
B.��ʹƷ����Һ��ɫ�����ʲ�һ����SO2
C.SO2��Ư�ۡ�����̿��Na2O2����ʹ��īˮ��ɫ����ԭ����ͬ
D.�����ʵ�����SO2��Cl2��Ϻ�ͨ��װ��ʪ�����ɫ�����ļ���ƿ�У�Ư��Ч������
E.����Ũ�������SO2
F.���ó����ʯ��ˮ����SO2��CO2
(4) װ�ñ���ʢ�Լ�Ϊ_______����ȱ��װ���ң��������Ȼ���ʧ���÷�Ӧ�Ļ�ѧ����Ϊ______________��
(5) ����������Ҳ�����Ȼ���(ClSO3H)�ֽ��ã��÷�Ӧ�Ļ�ѧ����ʽΪ��2ClSO3H===H2SO4��SO2Cl2���˷����õ��IJ�Ʒ�л�������ᡣ
�ٴӷֽ�����з���������ȵķ�����______________��
�������ʵ�鷽�������Ʒ��������(��ѡ�Լ���ϡ���ᡢϡ���ᡢBaCl2��Һ������ˮ��ʯ����Һ)____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��4���������������Ԫ�ص����λ�������Ԫ��X��ԭ�Ӻ����������M��2����Y��������������ԡ��ش��������⣺
![]()
��1��Ԫ��X�����ڱ��е�λ���ǵ�________���ڡ���________�壬�䵥�ʿɲ��õ������________�ķ����Ʊ���
��2��M��N��Y����Ԫ������������Ӧ��ˮ�����У�������ǿ����____________��������ǿ����______��(�ѧʽ)
��3���������(MN)2�ĵ���ʽΪ___________��(MN)2��Ϊ��±�أ�������±�����ƣ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com