
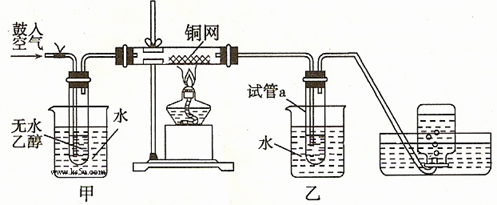
 ������д��ѧʽ�����ʽ��
������д��ѧʽ�����ʽ��  2CuO CH3CH2OH+CuO
2CuO CH3CH2OH+CuO CH3CHO+Cu+H2O ���ȷ�Ӧ
CH3CHO+Cu+H2O ���ȷ�Ӧ

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2Fe2O3+8SO2����������ʵ�飬���ⶨ����Ʒ��FeS2��Ʒ�Ĵ��ȣ������������ʲ����뷴Ӧ����
2Fe2O3+8SO2����������ʵ�飬���ⶨ����Ʒ��FeS2��Ʒ�Ĵ��ȣ������������ʲ����뷴Ӧ����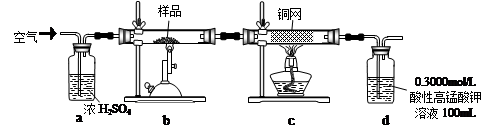
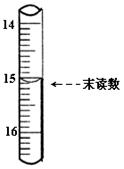
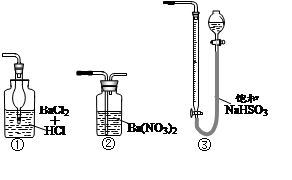
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
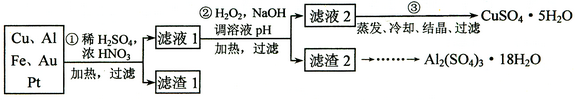
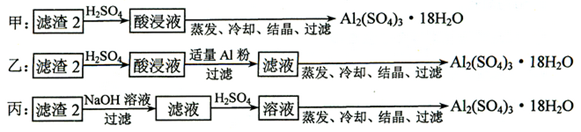
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������KMnO4��Һ������ϩ����Ȳ |
| B������м����ˮ����������屽 |
| C���ڱ������������÷�Һ�ķ������� |
| D����ͭ˿�ھƾ����ϼ��Ⱥ�����������ˮ�Ҵ��У�ͭ˿�ָ���ԭ���ĺ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
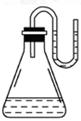
| A��NaOH���� | B��ŨH2SO4 | C��NH4NO3���� | D��Na2O2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ���� | I�C�ij�ʼŨ�� (mol��L-1) | OCl�C�ij�ʼŨ�� (mol��L-1) | OH�C�ij�ʼŨ�� (mol��L-1) | ��ʼ����v (mol��L-1�� s-1) |
| 1 | 2 �� 10�C3 | 1.5 �� 10�C3 | 1.00 | 1.8 �� 10�C4 |
| 2 | a | 1.5 �� 10�C3 | 1.00 | 3.6 �� 10�C4 |
| 3 | 2 �� 10�C3 | 3 �� 10�C3 | 2.00 | 1.8 �� 10�C4 |
| 4 | 4 �� 10�C3 | 3 �� 10�C3 | 1.00 | 7.2 �� 10�C4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
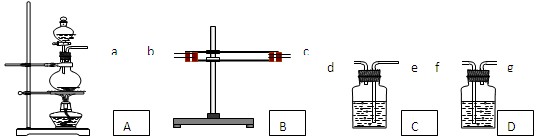
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| B����ȡ��ˮ�еĵⵥ��ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� |
| C����PH��ֽ�ⶨij��ɫ��Һ��PHʱ��Ӧ��PH��ֽ������Һ�У��۲�����ɫ�仯��������ɫ���Ƚ� |
| D����������У��¶ȼ�Ӧ�÷���������ƿ�ڵ�Һ���У��Բ���Һ����¶� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com