
�������(K2FeO4)�����Ͷ��ˮ�����������������������ȶ����������������£�
��ش���������
��1���ȼҵ��Cl2�Ļ�ѧ��Ӧ����ʽ ��
��2�����ɡ���ӦҺ�����ӷ���ʽ�� ����3��ʹ�ü���KClO��ԭ���� ��
��4���ӡ���ӦҺII���з����K2FeO4�� ������Ʒ��KCl�� (�ѧʽ)���û������� ���������ᴿ������ĸ��ţ���
| A������ | B����Һ | C������ | D���ؽᾧ |
��1��2NaCl + 2H2O 2NaOH + H2��+ Cl2��
2NaOH + H2��+ Cl2��
��2��3ClO- + 2Fe3+ + 10OH- = 2FeO42- + 3Cl- + 5H2O��
��3��K2FeO4�ڼ����������ȶ���KClO������ǿ��K2FeO4
��4�� KNO3 �� D
��5�� 3.00 �� 104
���������������1���ȼҵ���ǵ�ⱥ��ʳ��ˮ�õ��ռ����������2���������̷�����ӦҺII�����ô���������������ӵõ�FeO42-�����������ԭ��Ӧ���ӷ���ʽ����д����ɵã�3ClO- + 2Fe3+ + 10OH- = 2FeO42- + 3Cl- + 5H2O����3����Ŀ��Ϣ��K2FeO4�ڼ����������ȶ�����Ӧ���ڼ��������½��У���4����Ӧ�м����������������Ի�����KNO3������ص��ܽ�����¶ȱ仯�ϴ�������ȴ�ᾧ�ķ������룻��5������������Ӧ�ʹ����������������Ӧ�Ĺ�ϵʽ���м��㡣
���㣺�����ɢϵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���������̿�MnO2Լ70����Al2O3������п��ZnSԼ80����FeS������ͬ����MnO2��Zn���ɵ��ԭ�ϣ���
��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O MnO2+Zn+2H2SO4��
MnO2+Zn+2H2SO4��
��1��A�����ڻ�ԭ������� ��
��2��MnCO3��Zn2(OH)2CO3�������� ������Ҫ���ȵ�ԭ���� ��C�Ļ�ѧʽ�� ��
��3�����з��������ӷ���ʽΪ �� ��
��4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú���A1203��SiO2������FeO��xFe2O3�������Ʊ�A12(SO4)3��18H2O�������������£�
��һ�������£�MnO4 - ����Mn2+��Ӧ����MnO2��
��֪�������������������pH
| | Al��OH��3 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����ʱ | 3��4 | 6��3 | 2��7 |
| ��ȫ����ʱ | 5��2 | 9��7 | 3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����÷���м�����������������������ȣ�������ʽ������[Fe(OH)SO4]�Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(CeO2)��һ����Ҫ��ϡ�������ƽ�������ʾ�����������в��������ķϲ�����ĩ(��SiO2��Fe2O3��CeO2�Լ���������������ϡ�������)��ij�������Դ˷�ĩΪԭ�ϻ����棬���ʵ����������: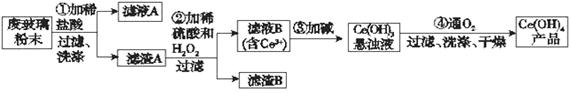
��1��ϴ������A��Ŀ����Ϊ�˳�ȥ____________�������ӷ��ţ�������������Ƿ�ϴ���ķ�����_________________________________________________________��
��2���ڢڲ���Ӧ�����ӷ���ʽ��______________________________________������B����Ҫ�ɷ���___________________��
��3����ȡ�Ƿ���ϡ��Ԫ�صij��÷�������֪������TBP��Ϊ��ȡ���ܽ������Ӵ�ˮ��Һ����ȡ������TBP______________����ܡ����ܡ�����ˮ���ܡ�ʵ���ҽ�����ȡ����ʱ�õ�����Ҫ����������_______________���ձ�������������Ͳ�ȡ�
��4��ȡ���������еõ���Ce(OH)4��Ʒ0.536 g���������ܽ����0.100 0 mol��L��1 FeSO4����Һ�ζ����յ�ʱ���汻��ԭΪCe3����������25.00 mL����Һ���ò�Ʒ��Ce(OH)4����������Ϊ___________�����������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����пΪ��ɫ��ĩ��������ʪ�Ѣ��Ƥ���������ơ�������ҵ������п[����Fe(��)��Mn(��)��Ni(��)������]���������£�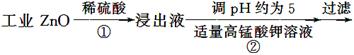
��ʾ���ڱ�ʵ�������£�Ni(��)���ܱ�������������صĻ�ԭ������MnO2��
�ش��������⣺
(1)��Ӧ���г���������������__________��������Ӧ�����ӷ���ʽΪ__________���ڼӸ��������Һǰ����pH�ϵͣ��Գ��ӵ�Ӱ����________________��
(2)��Ӧ�۵ķ�Ӧ����Ϊ____________�����˵õ��������У����˹�����п���______________��
(3)��Ӧ���γɵij���Ҫ��ˮϴ����������Ƿ�ϴ�Ӹɾ��ķ�����________________��
(4)��Ӧ���в���ijɷֿ�����ZnCO3��xZn(OH)2��ȡ�������˱�11.2 g�����պ�ɵõ���Ʒ8.1 g����x����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ϡ��Ԫ�������ڱ��Т� B���֡��ƺ���ϵԪ�ص��ܳƣ����Ƕ��Ǻܻ��õĽ��������ʼ�Ϊ���ƣ��������ϼ�Ϊ+3�������ƣ�Y��Ԫ���Ǽ���ͳ�������Ҫ���ϡ��ҹ��̲��ŷḻ���ƿ�ʯ�� Y2FeBe2Si2O10�����Դ˿�ʯΪԭ�����������ƣ�Y2O3������Ҫ�������£�
��֪��I���йؽ��������γ������������ʱ��pH���±���
| | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe3+ | 2.7 | 3.7 |
| Y3+ | 6.0 | 8.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�DZ������Ȼ��Դ�����ú�ˮˮ���Եõ�һϵ�в�Ʒ��Ҳ���Խ��з���������
��1�������ȼҵ��Ʒ������SO2���������������£�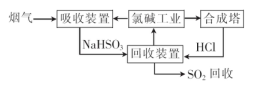
�١�����װ�á��з�����Ӧ�����ӷ���ʽ�� .
������������ѭ�����õ������� ��
��2�����ú�ˮ���������Ч�ؽ��úȼ���ŷŵ�SO2��ɵ�һϵ�л������⡣�乤��������ͼ��ʾ��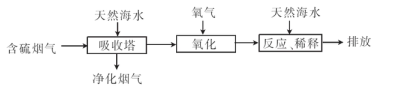
��Ȼ��ˮ���պ������������Ҫ���������������������䷴Ӧԭ���Ļ�ѧ����ʽ�� ��������ĺ�ˮ��Ҫ�����������ƣ���֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
��3���Ӻ�ˮ�������κ��ĸҺ�к���K+��Na+��Mg2+�������ӣ���ĸҺ����һϵ�еļӹ����Ƶý���þ��
�ٴ����ӷ�ϯ�ĽǶ�˼������ĸҺ�м���ʯ��������������� ��
��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ�� ��
�۵�����ڵ���ˮ�Ȼ�þ���õ�þ�������ض��Ļ�������ȴ��Ϊ����þ�����������п�������þ��������ȴ������ ������ĸ����
A��Ar B��CO2 C ���� D��O2 E��ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
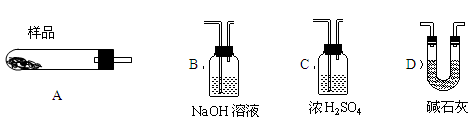
����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
��1������ʯ��������ܽ����Һ�����Mg2+�⣬�����еĽ��������� ��
��2�����Т����ʱ��������Һ��pH=7~8���й��������������pH���±�����Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ�� �ܽ⣬���� ������
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com