������Ҫ�Ļ���ԭ�ϣ��ϳɰ��ǹ���1905�귢���ģ�2001��ϣ����ѧ������˵��ϳɰ��ķ��������ڳ�ѹ�°��������ú���ϡ�͵ĵ����ֱ�ͨ��һ�����ȵ�570��ĵ����У�H
2��N
2�ڵ缫�Ϻϳɰ����˷���ת���ʴ�Ϊ��ߣ���֪��N
2 ��g��+3H
2 ��g��?2NH
3��g����H��0����ش��������⣺
��1����ⷨ�ϳɰ�����ʹ��ˮ�����Һ��
��Ϊʲô��
��
��2�����ϳɰ���Ӧ�������ݻ�Ϊ2L����ʼ����3mol H
2��1mol N
2 ��Ӧ�ﵽƽ��ʱ�����ѹǿ��Ϊԭ����
��������ת����Ϊ
���÷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽΪ
�������¶ȣ�ƽ�ⳣ����
�����������ٻ䣩������Ӧ������������ٴﵽƽ��ʱ���������ƽ����������
�����������ٻ䣩��
��3����һ�������°������������ʷ�Ӧ�������ء�CO��NH
2��
2������֪30g�����ڸ��¸�ѹ�·ֽ�����11.2LNH
3���������5.6LCO
2��һ�����������谷���ӣ������谷����Է�������Ϊ126���������������ڵ͵��������̷��м��������谷������̷��еĵ����ʺ�������������Ӥ������ʯ��д�������谷�ķ���ʽΪ
���仯ѧ��Ӧ����ʽΪ
�� �����谷��������һ����Ԫ����һ�������谷���ӿɷֽ�������������A����A�Ľṹʽ��
��


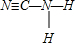 ��
��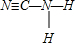 ��
��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
 ������Ҫ�Ļ���ԭ�ϣ�ʵ������ȡ�����Ļ�ѧ����ʽΪ
������Ҫ�Ļ���ԭ�ϣ�ʵ������ȡ�����Ļ�ѧ����ʽΪ ������Ҫ�Ļ���ԭ�ϣ�ʵ������ȡ�����Ļ�ѧ����ʽΪ________����ͼ��ʾ��A��B��C�������ռ�װ�ã����п������ռ���������________���������ֱ�պ��Ũ��ˮ��Ũ����IJ���������ʱ����۲쵽��������________����ʵ���ң����ﰱʱ��ͨ���ǽ��Ƶõİ�ͨ��________���Գ�ȥ���е�ˮ������Ϊ���鰱���Ƿ��ռ���������ȡ________��ֽ�����Թܿڴ�������ɫ��________ ʱ�������Ѿ��ռ���������
������Ҫ�Ļ���ԭ�ϣ�ʵ������ȡ�����Ļ�ѧ����ʽΪ________����ͼ��ʾ��A��B��C�������ռ�װ�ã����п������ռ���������________���������ֱ�պ��Ũ��ˮ��Ũ����IJ���������ʱ����۲쵽��������________����ʵ���ң����ﰱʱ��ͨ���ǽ��Ƶõİ�ͨ��________���Գ�ȥ���е�ˮ������Ϊ���鰱���Ƿ��ռ���������ȡ________��ֽ�����Թܿڴ�������ɫ��________ ʱ�������Ѿ��ռ���������
