
ЁОЬтФПЁПЕкЫФжмЦкЕФдЊЫиЃЌШчЃКюб(22Ti)ЁЂЬњ(26Fe)ЁЂЩщЁЂЮјЁЂаПЕШМАЦфЯрЙиЛЏКЯЮядкЛЏЙЄЁЂвНвЉЁЂВФСЯЕШСьгђгазХЙуЗКЕФгІгУЁЃЛиД№ЯТСаЮЪЬтЃК
(1)ЛљЬЌTiдзгжаЃЌзюИпФмВуЕчзгЕФЕчзгдЦТжРЊаЮзДЮЊ___________ЃЌгыTiЭЌжмЦкЕФЫљгаЙ§ЖЩдЊЫиЕФЛљЬЌдзгжаЃЌзюЭтВуЕчзгЪ§гыюбВЛЭЌЕФдЊЫига______жжЁЃ
(2)чњчъЫсбЧЬњЦЌЪЧгУгкШБЬњадЦЖбЊЕФдЄЗРКЭжЮСЦЕФГЃМћвЉЮяЃЌСйДВНЈвщЗўгУЮЌЩњЫиCДйНјЁАбЧЬњЁБЕФЮќЪеЃЌБмУтЩњГЩFe3+ЃЌДгНсЙЙНЧЖШРДПДЃЌFe2+ взБЛбѕЛЏГЩFe3+ЕФдвђЪЧ______________ЁЃ
(3)SCN-РызгПЩгУгкFe3+ЕФМьбщЃЌЦфЖдгІЕФЫсгаСНжжЃЌЗжБ№ЮЊСђЧшЫс(H-S-CЁдN)КЭвьСђЧшЫс(H-N=C=S)ЁЃ
ЂйаДГігыSCN-ЛЅЮЊЕШЕчзгЬхЕФвЛжжЮЂСЃ_________________(ЗжзгЛђРызг)ЃЛ
ЂкСђЧшЫсЗжзгжаІаМќКЭІвМќЕФИіЪ§жЎБШЮЊ___________ЃЛ
ЂлвьСђЧшЫсЕФЗаЕуБШСђЧшЫсЗаЕуИпЕФдвђЪЧ________________________ЁЃ
(4)ГЩгяЁАаХПкДЦЛЦЁБжаЕФДЦЛЦЗжзгЪНЮЊAs2S3ЃЌЗжзгНсЙЙШчЭМЃЌAsдзгЕФдгЛЏЗНЪНЮЊ____________ЃЌДЦЛЦКЭSnCl2дкбЮЫсжаЗДгІзЊЛЏЮЊДЦЛЦЃЈAs4S4ЃЉКЭSnCl4ВЂЗХГіH2SЦјЬхЃЌаДГіИУЗДгІЗНГЬЪН__________________________ЁЃSnCl4ЗжзгЕФПеМфЙЙаЭЮЊ______________ЁЃ
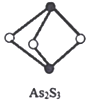
(5)ИпзгЛЏКЯЮяCaC2ЕФвЛжжОЇЬхНсЙЙШчЭМЫљЪОЁЃИУЮяжЪЕФЕчзгЪН___________ЁЃвЛИіОЇАћКЌгаЕФІаМќЦНОљга___________ИіЁЃ
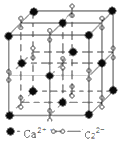
(6)ЮјЛЏаПЕФОЇАћНсЙЙШчЭМЫљЪОЃЌЭМжаXКЭYЕуЫљЖбЛ§ЕФдзгОљЮЊ___________(ЬюдЊЫиЗћКХ)ЃЛИУОЇАћжаЮјдзгЫљДІПеЯЖРраЭЮЊ___________(ЬюЁАСЂЗНЬхЁБЁЂЁАе§ЫФУцЬхЁБЛђе§АЫУцЬхЁБ)ЃЌИУжжПеЯЖЕФЬюГфТЪЮЊ___________ЃЛШєИУОЇАћУмЖШЮЊpgcm-3ЃЌЮјЛЏаПЕФФІЖћжЪСПЮЊMgmol-1ЁЃгУNAДњБэАЂЗќМгЕТТоГЃЪ§ЕФЪ§жЕЃЌдђОЇАћВЮЪ§a ЮЊ___________nmЁЃ
ЁОД№АИЁП ЧђаЮ 2 Fe3+ЕФ3d5АыТњзДЬЌИќЮШЖЈ N2O(ЛђCO2ЁЂCS2ЁЂOCN-) 2ЁУ3 вьСђЧшЫсЗжзгМфКЌгаЧтМќ sp3дгЛЏ 2As2S3+2SnCl2+4HCl=As4S4+2SnCl4+2H4SЁќ е§ЫФУцЬхаЮ ![]() 8 Zn е§ЫФУцЬх 50%
8 Zn е§ЫФУцЬх 50% ![]() ЁС107
ЁС107
ЁОНтЮіЁП(1)ЛљЬЌTiдзгЕФМлВуЕчзгХХВМЪНЮЊЃК3d24s2ЃЌзюИпФмВуЮЊЕкЫФФмВуЃЌsЕчзгдЦТжРЊаЮзДЮЊЧђаЮЃЛгыTiЭЌжмЦкЕФЫљгаЙ§ЖЩдЊЫиЕФЛљЬЌдзгжаЃЌзюЭтВуЕчзгЪ§гыюбВЛЭЌЕФдЊЫигаCr 3d54s1ЃЌCu 3d104s1ЃЌЙВСНжжЃЛ
ЃЈ2ЃЉДгНсЙЙНЧЖШРДПДЃЌFe2+ ЕФМлЕчзгХХВМЪНЪЧЃК3d6ЃЌдйЪЇвЛИіЕчзгОЭЪЧ3d5АыГфТњЮШЖЈНсЙЙЃЌЙЪвзБЛбѕЛЏГЩFe3+ЃЛ
(3) ЂйгыSCN-ЛЅЮЊЕШЕчзгЬхЕФвЛжжЮЂСЃЪЧЃКN2O(ЛђCO2ЁЂCS2ЁЂOCN-)ЃЛ
ЂкСђЧшЫс(H-S-CЁдN)жаІаМќКЭІвМќЕФИіЪ§жЎБШЮЊ2ЁУ3ЃЛ
ЂлвьСђЧшЫсЗжзгМфПЩвдаЮГЩЧтМќЃЌЙЪЦфЗаЕуБШСђЧшЫсЗаЕуИпЃЛ
(4) ДЦЛЦЗжзгЪНЮЊAs2S3ЃЌAsгаЫФЖдМлВуЕчзгЖдЃЌAsдзгЕФдгЛЏЗНЪНЮЊsp3дгЛЏЃЛ
ДЦЛЦКЭSnCl2дкбЮЫсжаЗДгІзЊЛЏЮЊДЦЛЦЃЈAs4S4ЃЉКЭSnCl4ВЂЗХГіH2SЦјЬхЃЌЙЪЦфЗДгІЗНГЬЪНЮЊЃК2As2S3+2SnCl2+4HCl=As4S4+2SnCl4+2H4SЁќЃЛSnCl4ЗжзгЕФПеМфЙЙаЭЮЊе§ЫФУцЬхЃЛ
(5)CaC2ЮЊРызгЛЏКЯЮяЃЌЕчзгЪНЮЊЃК![]() ЃЛвЛИіОЇАћКЌга4ИіCa2+ЃЌ4ИіC22-,вЛИіC22-жагаСНИіІаМќЃЌвЛИіОЇАћКЌгаЕФІаМќЦНОљга8ИіЃЛ
ЃЛвЛИіОЇАћКЌга4ИіCa2+ЃЌ4ИіC22-,вЛИіC22-жагаСНИіІаМќЃЌвЛИіОЇАћКЌгаЕФІаМќЦНОљга8ИіЃЛ
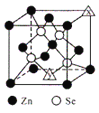
(6) ЮјЛЏаПЕФОЇАћНсЙЙжаXКЭYЕуЫљЖбЛ§ЕФдзгОљЮЊZnЃЌИУОЇАћжаЫФИіЮјдзгЫљДІОЇАћЬхЖдНЧЯпЫФЗжжЎвЛДІЃЌПеЯЖРраЭЮЊе§ЫФУцЬхЃЌЕЋжЛеМОнСЫЦфжа4ИіЮЛжУЃЌЙЪИУжжПеЯЖЕФЬюГфТЪЮЊ50%ЃЛШєИУОЇАћУмЖШЮЊpgcm-3ЃЌЮјЛЏаПЕФФІЖћжЪСПЮЊMgmol-1ЁЃгУNAДњБэАЂЗќМгЕТТоГЃЪ§ЕФЪ§жЕЃЌдђОЇАћВЮЪ§a ЮЊ![]() ЁС107nmЁЃ
ЁС107nmЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП25ЁцЪБ,гУ0.10m/LЕФШѕМюBOHЕЮЖЈ10.00 ml a mol/LЕФбЮЫс,ШмвКЕФpHгыЫљМгBOHШмвКЬхЛ§(V)ЕФЙиЯЕШчЭМЫљЪОЁЃвбжЊNЕуШмвКжаДцдкЙиЯЕЪН:c(C1ЁЊ)=c(B+)+c(BOH)ЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
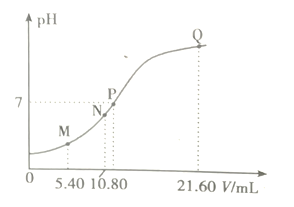
A. MЕуШмвКжаДцдкc(H+)=c(OHЁЊ)+2c(BOH)+c(B+)
B. NЁЂQСНЕуШмвКжаBOHЕчРыЦНКтГЃЪ§:N<Q
C. PЕуBOHЙ§СП,ДЫЪБШмвКжаc(B+)>c(ClЁЊ)
D. a<0.108
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПХ№Ыс(H3BO3)ЮЊвЛдЊШѕЫсЃЌвбжЊH3BO3гызуСПNaOHШмвКЗДгІЕФРызгЗНГЬЪНЮЊH3BO3+OH-=B(OH)4-ЃЌH3BO3ПЩвдЭЈЙ§ЕчНтЕФЗНЗЈжЦБИЁЃЦфЙЄзїдРэШчЯТЭМЫљЪО(бєФЄКЭвѕФЄЗжБ№жЛдЪаэбєРызгЁЂвѕРызгЭЈЙ§)ЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧЃЈ ЃЉ
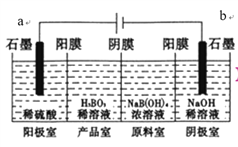
A. aгыЕчдДЕФе§МЋЯрСЌНг
B. бєМЋЕФЕчМЋЗДгІЪНЮЊЃК2H2O-4e-=O2Ёќ+4H+
C. ЕБЕчТЗжаЭЈЙ§3molЕчзгЪБЃЌПЩЕУЕН1molH3BO3
D. B(OH)4-ДЉЙ§вѕФЄНјШыВњЦЗЪвЃЌNa+ДЉЙ§бєФЄНјШывѕМЋЪв
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЂёЃЎДпЛЏМСЪЧЛЏЙЄММЪѕЕФКЫаФЃЌОјДѓЖрЪ§ЕФЛЏЙЄЩњВњашВЩгУДпЛЏЙЄвеЁЃ
ЃЈ1ЃЉаТЕФбаОПБэУїЃЌПЩвдНЋCO2зЊЛЏЮЊЬПКкНјааЛиЪеРћгУЃЌЗДгІдРэШчЭМЫљЪОЁЃ
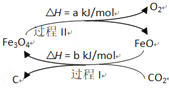
ећИіЙ§ГЬжаFe3O4ЕФзїгУЪЧ________________________________ЁЃ
ЃЈ2ЃЉвбжЊЗДгІN2(g)+3H2(g)![]() 2NH3(g) ІЄHЃМ0ЁЃАДn(N2)ЁУn(H2) = 1ЁУ3ЯђЗДгІШнЦїжаЭЖСЯЃЌдкВЛЭЌЮТЖШЯТЗжБ№ДяЦНКтЪБЃЌЛьКЯЦјжаNH3ЕФжЪСПЗжЪ§ЫцбЙЧПБфЛЏЕФЧњЯпШчЭМЫљЪОЃК
2NH3(g) ІЄHЃМ0ЁЃАДn(N2)ЁУn(H2) = 1ЁУ3ЯђЗДгІШнЦїжаЭЖСЯЃЌдкВЛЭЌЮТЖШЯТЗжБ№ДяЦНКтЪБЃЌЛьКЯЦјжаNH3ЕФжЪСПЗжЪ§ЫцбЙЧПБфЛЏЕФЧњЯпШчЭМЫљЪОЃК
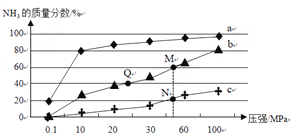
ЂйЯТСаЫЕЗЈе§ШЗЕФЪЧ__________ЁЃЃЈЬюзжФИЃЉ
aЃЎЧњЯпaЁЂbЁЂcЖдгІЕФЮТЖШЪЧгЩЕЭЕНИп
bЃЎМгШыДпЛЏМСФмМгПьЛЏбЇЗДгІЫйТЪКЭЬсИпH2ЕФзЊЛЏТЪ
cЃЎЭМжаQЁЂMЁЂNЕуЕФЦНКтГЃЪ§ЃКK(N)ЃОK(Q)=K(M)
ЂкMЕуЖдгІH2ЕФзЊЛЏТЪЪЧ__________ЁЃ
Ђл2007ФъЛЏбЇМвИёЙўЕТЁЄАЃЬиЖћдкЙўВЎбаОПЫљжЄЪЕСЫЧтЦјгыЕЊЦјдкЙЬЬхДпЛЏМСБэУцКЯГЩАБЕФЗДгІЙ§ГЬЃЌЪОвтЭМШчЯТЃК
![]() ЁЂ
ЁЂ![]() КЭ
КЭ![]() ЗжБ№БэЪОN2ЁЂH2КЭNH3ЁЃeБэЪОЩњГЩЕФNH3РыПЊДпЛЏМСБэУцЃЌbКЭcЕФКЌвхЗжБ№ЪЧ____________КЭ______________ЁЃ
ЗжБ№БэЪОN2ЁЂH2КЭNH3ЁЃeБэЪОЩњГЩЕФNH3РыПЊДпЛЏМСБэУцЃЌbКЭcЕФКЌвхЗжБ№ЪЧ____________КЭ______________ЁЃ
ЃЈ3ЃЉгаЛњЗДгІжавВГЃгУЕНДпЛЏМСЁЃФГЗДгІдРэПЩвдгУЯТЭМБэЪОЃЌаДГіДЫЗДгІЕФЛЏбЇЗНГЬЪН_____________________________ЁЃ
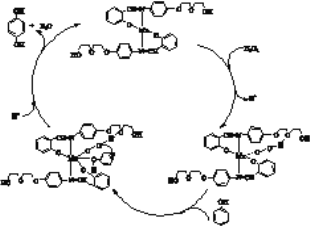
ЂђЃЎЙ§ЖўСђЫсМи(K2S2O8)дкПЦбагыЙЄвЕЩЯгаживЊгУЭОЁЃ
(4)ФГГЇВЩгУЪЊЗЈK2S2O8бѕЛЏЭбЯѕКЭАБЗЈЭбСђЙЄвезлКЯДІРэШМУКЙјТЏбЬЦјЃЌЬсИпСЫбЬЦјДІРэаЇТЪЃЌДІРэвКЛЙПЩвдгУзїГЧЪажВБЛТЬЛЏЕФЗЪСЯЁЃвЛЖЈЬѕМўЯТЃЌNOШЅГ§ТЪЫцЮТЖШБфЛЏЕФЙиЯЕШчЭМЫљЪОЁЃ80ЁцЪБЃЌШєNOГѕЪМХЈЖШЮЊ450 mgЁЄm-3ЃЌt minДяЕНзюДѓШЅГ§ТЪЃЌNOШЅГ§ЕФЦНОљЗДгІЫйТЪЃКv(NO)=__________molЁЄL-1ЁЄMin-1ЃЈСаДњЪ§ЪНЃЌВЛБиМЦЫуНсЙћЃЉ
(5)Й§ЖўСђЫсМиПЩЭЈЙ§ЁАЕчНтЁњзЊЛЏЁњЬсДПЁБЗНЗЈжЦЕУЃЌЕчНтзАжУЪОвтЭМШчЭМЫљЪОЁЃ
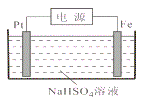
ГЃЮТЯТЃЌЕчНтвКжаКЌСђЮЂСЃЕФжївЊДцдкаЮЪНгыpHЕФЙиЯЕШчЯТЭМЫљЪОЁЃ
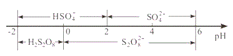
дкбєМЋЗХЕчЕФРызгжївЊЪЧHSO4ЃЌбєМЋЧјЕчНтжЪШмвКЕФpHЗЖЮЇЮЊ___________ЃЌбєМЋЕФЕчМЋЗДгІЪНЮЊ_____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЂёЃЎЁАвЛЫсСННўЃЌСНМюСЊКЯЁБЗЈЪЧЪЕЯжЗлУКЛвЃЈКЌSiO2ЁЂAl2O3ЁЂFe2O3ЁЂCaOЁЂMgOЕШЃЉзлКЯРћгУЕФаТЙЄвеЁЃОлКЯТШЛЏТСЬњЃЈPAFCЃЉЛЏбЇЪНЮЊЃК[Al2(OH)nCl6Ѓn]mЁЄ[Fe2(OH)xCl6Ѓx]yЃЌЪЧвЛжжаТаЭИпаЇЕФОЛЫЎМСЁЃЙЄвЕСїГЬШчЯТЃК

ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЪЕМЪЙЄвЕжаЁАЫсНўЁБЁЂЁАМюНўЁБОљВЛФмГфЗжЗДгІЃЌТЫдќAжажївЊКЌгаSiO2ЁЂAl2O3ЁЃЁАДПМюЛьКЯБКЩеЁБжаЃЌЫќУЧЗжБ№ЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________________ЁЂ_________________ЁЃ
ЃЈ2ЃЉТЫвКЛьКЯКѓЁАеєЗЂЁБЕФзїгУЪЧ________________________________ЁЃ
ЃЈ3ЃЉЁАЕчНтЁБЪБбєМЋЕФЕчМЋЗДгІЪНЮЊ________________ЁЃ
ЃЈ4ЃЉPAFCЁЂЮоЫЎAlCl3ЁЂFeCl3ЁЄ6H2OгУСПОљЮЊ25 mgЁЄL-1ЪБЃЌДІРэВЛЭЌpHЮлЫЎЕФзЧЖШШЅГ§ТЪШчЭМЫљЪОЃЌPAFCЕФгХЕуЪЧ___________________________ЁЃ
ЂђЃЎ(5)25ЁцЪБЃЌгУ0.1mol/LЕФCH3COOHШмвКЕЮЖЈ20mL0.1mol/LЕФNaOHШмвКЃЌЕБЕЮМгVmLCH3COOHШмвКЪБЃЌЛьКЯШмвКЕФpH=7ЁЃвбжЊCH3COOHЕФЕчРыЦНКтГЃЪ§ЮЊKaЃЌКіТдЛьКЯЪБШмвКЬхЛ§ЕФБфЛЏЃЌKaЕФБэДяЪНЮЊ___________ЁЃ
(6)ГЃЮТЯТЃЌВтЕУФГДПCaSO3гыЫЎаЮГЩЕФзЧвКpHЮЊ9ЃЌвбжЊKa1(H2SO3)ЃН1.8ЁС10-2ЃЌKa2(H2SO3)ЃН6.0ЁС10-9ЃЌКіТдSO32-ЕФЕкЖўВНЫЎНтЃЌдђKsp(CaSO3)ЃН_________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈе§ШЗЕФЪЧ (ЁЁЁЁ)
A. дзгзюЭтВуЕчзгЪ§ЮЊ2ЕФдЊЫивЛЖЈДІгкжмЦкБэЕкЂђAзх
B. жїзхдЊЫиXЁЂYФмаЮГЩXY2аЭЛЏКЯЮяЃЌдђXгыYЕФдзгађЪ§жЎВюПЩФмЮЊ2Лђ5
C. ТШЛЏЧтЕФЗаЕуБШЗњЛЏЧтЕФЗаЕуИп
D. ЭЌжїзхдЊЫиаЮГЩЕФбѕЛЏЮяЕФОЇЬхРраЭОљЯрЭЌ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
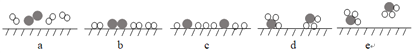
ЁОЬтФПЁПжьздЧхдкЁЖКЩЬСдТЩЋЁЗжааДЕРЃКЁАБЁБЁЕФЧрЮэИЁЦ№дкКЩЬСРяЁЁдТЙтЪЧИєСЫЪїееЙ§РДЕФЃЌИпДІДдЩњЕФЙрФОЃЌТфЯТВЮВюЕФАпВЕЕФКкгАЁЁЁБдТЙтДЉЙ§БЁЮэаЮГЩЕФжжжжУРОАБОжЪдвђЪЧ
A.ЮэЪЧвЛжжНКЬхB.ПеЦјжаЕФаЁЫЎЕЮПХСЃЕФВМРЪдЫЖЏ
C.ЗЂЩњЖЁДяЖћЯжЯѓD.ПеЦјжаЕФаЁЫЎЕЮПХСЃжБОЖДѓаЁдМЮЊ1ЁЋ100 nm
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
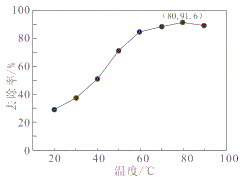
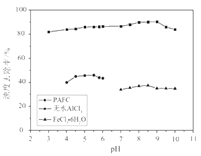
ЁОЬтФПЁПЫцзХВФСЯПЦбЇЕФЗЂеЙЃЌН№ЪєЗАМАЦфЛЏКЯЮяЕУЕНСЫдНРДдНЙуЗКЕФгІгУ,ВЂБЛгўЮЊЁАКЯН№ЮЌЩњЫиЁБЁЃЙЄвЕЩЯЛиЪеЗЯЗАДпЛЏМСЃЈКЌгаV2O5ЁЂVOSO4МАВЛШмадВадќЃЉЃЌПЦбаШЫдБзюаТбажЦСЫвЛжжРызгНЛЛЛЗЈЛиЪеЗАЕФаТЙЄвеЃЌЛиЪеТЪДя91ЃЎ7%вдЩЯЁЃИУЙЄвеЕФжївЊСїГЬШчЭМЫљЪОЃК
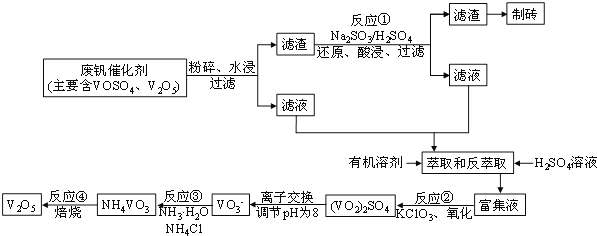
вбжЊВПЗжКЌЗАЮяжЪдкЫЎжаЕФШмНтадШчЯТБэЫљЪОЃК
ЮяжЪ | VOSO4 | V2O5 | NH4VO3 | ЃЈVO2ЃЉ2SO4 |
ШмНтад | ПЩШм | ФбШм | ФбШм | взШм |
ЧыЮЪД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЙЄвЕЩЯгЩV2O5вБСЖН№ЪєЗАГЃгУТСШШМСЗЈЃЌЦфЛЏбЇЗНГЬЪНПЩБэЪОЮЊ____ЁЃ
ЃЈ2ЃЉТЫвКжаКЌЗАЕФжївЊГЩЗж________ЃЈаДЛЏбЇЪНЃЉЁЃЗДгІЂйЕФРызгЗНГЬЪН___________________ЁЃ
ЃЈ3ЃЉнЭШЁКЭЗДнЭШЁЙ§ГЬжаЫљашЕФжївЊВЃСЇвЧЦїЮЊ_____________ЁЃШєЗДнЭШЁЪЙгУСђЫсгУСПЙ§ДѓЃЌНјвЛВНДІРэЛсдіМг_______ЃЈЬюЛЏбЇЪНЃЉЕФгУСПЃЌдьГЩГЩБОдіДѓЁЃ
ЃЈ4ЃЉИУЙЄвеЗДгІЂлЕФГСЕэТЪЃЈгжГЦГСЗАТЪЃЉЪЧЛиЪеЗАЕФЙиМќжЎвЛЃЌаДГіИУВНЗЂЩњЗДгІЕФРызгЗНГЬЪН___________________ЁЃЁАГСЕэЁБЙ§ГЬжаЃЌГСЗАТЪЪмЮТЖШЁЂТШЛЏяЇЯЕЪ§ЃЈNH4ClЕФжЪСПгыЕїНкpHжЎКѓЕФСЯвКжаVO3-ЕФжЪСПБШЃЉЕШЕФгАЯьЃЌЦфжаЮТЖШгыГСЗАТЪЕФЙиЯЕШчЭМЫљЪОЃЌЮТЖШИпгк80ЁцГСЗАТЪНЕЕЭЕФПЩФмдвђЪЧ___________________________ЁЃ

ЃЈ5ЃЉ25ЁцЪБЃЌШЁбљНјааЪдбщЗжЮіЃЌЕУЕНЗАГСЕэТЪКЭШмвКpHжЎМфЙиЯЕШчЯТБэЃК
pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
ЗАГСЕэТЪ% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
НсКЯЩЯБэЃЌдкЪЕМЪЩњВњжаЃЌЂлжаМгШыАБЫЎЃЌЕїНкШмвКЕФзюМбpHжЕЮЊ____ЁЃШєЗЏГСЕэТЪЮЊ93.1%ЪБВЛВњЩњFeЃЈOHЃЉ3ГСЕэЃЌдђШмвКжаcЃЈFe3+ЃЉЃМ_____ЁЃЃЈвбжЊЃК25ЁцЪБЃЌKsp[FeЃЈOHЃЉ3]ЃН2.6ЁС10-39ЃЉ
ЃЈ6ЃЉЗЯЗАДпЛЏМСжаV2O5ЕФжЪСПЗжЪ§ЮЊ6%ЃЈдСЯжаЕФЫљгаЗАвбЛЛЫуГЩV2O5ЃЉЁЃШЁ100gДЫЗЯЗАДпЛЏМСАДЩЯЪіСїГЬНјааЪЕбщЃЌЕБМгШы105mLЁЁ0.1molL-1ЕФKClO3ШмвКЪБЃЌШмвКжаЕФЗАЧЁКУБЛЭъШЋДІРэЃЌМйЩшвдКѓИїВНЗАУЛгаЫ№ЪЇЃЌдђИУЙЄвЕЩњВњжаЗАЕФЛиЪеТЪЪЧ______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАБЦјдкЙЄХЉвЕЩњВњКЭЙњЗРПЦММжагазХживЊгІгУЃЌПЦбаЙЄзїепЖдЦфНјааепЙуЗКбаОПЁЃЛиД№ЃК
(1)ФГПЮЬтзщЪЕЯжСЫдкГЃЪЊГЃбЙЯТ,вдЕЊЦјКЭвКЬЌЫЎЮЊдСЯжЦБИАБЦјЭЌЪБгабѕЦјЩњГЩЁЃ
вбжЊЃЌдквЛЖЈЮТЖШКЭбЙЧПЯТЃЌгЩзюЮШЖЈЕФЕЅжЪЩњГЩ1molДПЮяжЪЕФШШаЇгІЃЌГЦЮЊИУЮяжЪЕФЩњГЩШШ(ЁїH)ЁЃГЃЮТГЃбЙЯТЁЂЯрЙиЮяжЪЕФЩњГЩШШШчЯТБэЫљЪО:
ЮяжЪ | NH3(s) | H20(1) |
ЁїH/ kJЁЄmo1-1 | -46 | -242 |
ЩЯЪіКЯГЩАБЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ______________________ЁЃ
(2)РћгУЩњЮяЕчГиЃЌвдH2ЁЂN2ЮЊдСЯКЯГЩАБЕФзАжУШчЯТЭМЫљЪОЁЃ

QЁЂRОљЮЊДпЛЏМСЃЌОнЕНЪОХаЖЯЃЌИКМЋЗДгІЕФДпЛЏМСЮЊ___(ЬюЁАQЁБЛђЁАRЁБ)ЃЛе§МЋЕФЕчМЋЗДгІЪНЮЊ_______________ЁЃ
(3)АБЦјЪЧЙЄвЕжЦЯѕЫсЕФжївЊдСЯжЎвЛЃЌДпЛЏбѕЛЏВНжшжаЗЂЩњЕФжївЊЗДгІШчЯТЃК
I.4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)ЁїH=-906kJ/mol
4NO(g)+6H2O(g)ЁїH=-906kJ/mol
II.4NH3(g)+3O2(g)![]() 2N2(g)+ 6H2O(g)ЁїH=-126kJ/mol
2N2(g)+ 6H2O(g)ЁїH=-126kJ/mol
НЋЙЬЖЈБШР§NH3КЭO2ЕФЛьКЯЦјЬхвдвЛЖЈСїЫйЭЈЙ§ЬюГфгаДпЛЏМСЕФЗДгІЦїЃЌЗДгІВњТЪгыЮТЖШЕФЙиЯЕШчЭМ1ЫљЪОЁЃ
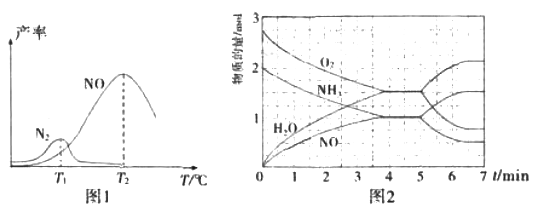
ЂйДпЛЏбѕЛЏВНжшжаЃЌзюЪЪвЫЕФЮТЖШЮЊ____(ЬюЁАT1ЁБЛђЁАT2ЁБ)ЁЃ
ЂкЕЭгкT1ЁцЪБЃЌNOЕФВњТЪНЯЕЭЕФдвђЮЊ_____ЁЃ
ЂлИпгкT2ЁцЪБЃЌNOЕФВњТЪНЕЕЭЕФПЩФмдвђЮЊ_____(ЬюбЁЯюзжФИ)
A.ДпЛЏМСЛюадНЕЕЭ B.ЦНКтГЃЪ§МѕаЁ C.ЗДгІЛюЛЏФмдіДѓ D.АБЦјШмгкЫЎ
ЂмT2Ёц(T1>T2)ЪБЃЌЯђ20LКуШнУмБеШнЦїжаГфШы2molNH3КЭ2.75mo1O2ЃЌЗЂЩњЗДгІI.ЗДгІЙ§ГЬжаИїЮяжЪЕФЮяжЪЕФСПЕФЫцЪБМф(t)БфЛЏЙиЯЕШчЭМ2ЫљЪОЁЃT2ЁцЪБЃЌИУЗДгІЕФЦНКтГЃЪ§K=_____ЃЛ5minЪБЃЌИФБфСЫФГвЛЭтНчЬѕМўЃЌЫљИФБфЕФЬѕМўПЩФмЮЊ__________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com