
ΓΨΧβΡΩΓΩœ¬±μ «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§«κΜΊ¥π”–ΙΊΈ ΧβΘΚ
÷ςΉε ÷ήΤΎ | ΔώA | ΔρA | ΔσA | ΔτA | ΔθA | ΔωA | ΔςA |
2 | ΔΌ | ΔΎ | Δέ | Δή | |||
3 | Δί | Δό | ΔΏ | Δύ | |||
4 | Δα | Δβ |
Θ®1Θ©ΒΎ2÷ήΤΎΒΡ8÷÷‘ΣΥΊ÷–Θ§ΒΎ“ΜΒγάκΡήΫι”ΎΔΌ‘ΣΥΊΚΆΔέ‘ΣΥΊΦδΒΡ‘ΣΥΊ”–__÷÷ΓΘ
Θ®2Θ©±μ÷–Ρή–Έ≥…ΝΫ–‘«β―θΜ·ΈοΒΡ‘ΣΥΊ «___Θ®Χν‘ΣΥΊΟϊ≥Τ![]() Θ§–¥≥ωΗΟ‘ΣΥΊΒΡΒΞ÷ ”κΔαΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ___ΓΘ
Θ§–¥≥ωΗΟ‘ΣΥΊΒΡΒΞ÷ ”κΔαΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ___ΓΘ
Θ®3Θ©ΔΎΓΔΔίΓΔΔΏΓΔΔαΝυ÷÷‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο÷–Θ§Α¥Φν–‘Φθ»θΥα–‘‘ω«ΩΒΡΥ≥–ρ≈≈Ν–ΈΣΘ®”ΟΜ·―ß Ϋ±μ ΨΘ©___ΓΘ
Θ®4Θ©Δή‘ΣΥΊ”κΔβ‘ΣΥΊΝΫ’Ώ÷ Ή” ΐ÷°≤ν «___ΓΘ
Θ®5Θ©«κ–¥≥ωΔέΒΡΤχΧε«βΜ·ΈοΖΔ…ζ¥ΏΜ·―θΜ·ΒΡΜ·―ßΖΫ≥Χ Ϋ___ΓΘ
Θ®6Θ©«κ–¥≥ωΔό‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο”κΔύ‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ___ΓΘ
ΓΨ¥πΑΗΓΩ3 ¬Ν 2Al+OH-+2H2O=2AlO2-+3H2Γϋ KOHΓΔMg(OH)2ΓΔH2CO3ΓΔH2SO4 26 4NH3+5O2 4NO+6H2O Al(OH)3+3H+=Al3++3H2O
4NO+6H2O Al(OH)3+3H+=Al3++3H2O
ΓΨΫβΈωΓΩ
(1)ΔΌΈΣAlΘ§ΔέNΘ§Ά§“Μ÷ήΤΎ‘ΣΥΊ÷–Θ§‘ΣΥΊΒΡΒΎ“ΜΒγάκΡήΥφΉ≈‘≠Ή”–ρ ΐΒΡ‘ω¥σΕχ≥ ‘ω¥σΒΡ«ς ΤΘ§Mg‘≠Ή”ΉνΆβ≤ψΈΣ»Ϊ¬ζΉ¥Χ§Θ§Υυ“‘ΒΎ“ΜΒγάκΡή¥σ”ΎAlΘ§N‘≠Ή”ΉνΆβ≤ψΈΣΑκ¬ζΉ¥Χ§Θ§Υυ“‘ΒΎ“ΜΒγάκΡή¥σ”ΎOΘ§Υυ“‘ΒΎ“ΜΒγάκΡήΫι”ΎAlΚΆNΒΡ‘ΣΥΊ”–MgΓΔCΓΔOΘ§Ι≤3÷÷ΘΜ
(2)¬Ν‘ΣΥΊΡή–Έ≥…ΝΫ–‘«β―θΜ·ΈοΘΜΔαΈΣKΘ§ΤδΉνΗΏΦέ―θΜ·ΒΡΥ°Μ·ΈοΈΣKOHΘ§AlΩ…“‘”κ«ΩΦνΖ¥”Π…ζ≥…«βΤχΚΆΤΪΥαΗυ―ΈΘ§Υυ“‘άκΉ”ΖΫ≥Χ ΫΈΣ2Al+OH-+2H2O=2AlO2-+3H2ΓϋΘΜ
(3)ΔΎΓΔΔίΓΔΔΏΓΔΔαΖ÷±πΈΣCΓΔMgΓΔSΓΔKΘ§Ϋπ τ–‘‘Ϋ«ΩΘ§ΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΒΡΦν–‘‘Ϋ«ΩΘ§Ϋπ τ–‘K>Mg>C>SΘ§Υυ“‘Φν–‘Φθ»θΥα–‘‘ω«ΩΒΡΥ≥–ρ≈≈Ν–ΈΣKOHΓΔMg(OH)2ΓΔH2CO3ΓΔH2SO4ΘΜ
(4)ΔήΈΣFΈΣ9Κ≈‘ΣΥΊΤδ‘≠Ή”Κ§9Ηω÷ Ή”Θ§ΔβΈΣBrΘ§ΈΣ35Κ≈‘ΣΥΊΤδ‘≠Ή”Κ§35Ηω÷ Ή”Θ§÷ Ή” ΐ÷°≤νΈΣ26ΘΜ
(5)ΔέΈΣNΘ§ΤδΤχΧε«βΜ·ΈοΈΣNH3Θ§¥ΏΜ·―θΜ·…ζ≥…NOΚΆΥ°Θ§ΖΫ≥Χ ΫΈΣ4NH3+5O2 4NO+6H2OΘΜ
4NO+6H2OΘΜ
(6)ΔόΈΣAlΘ§ΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΈΣAl(OH)3Θ§ΔύΈΣClΘ§ΉνΗΏΦέ―θΜ·ΈΣΈΣHClO4Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚAl(OH)3+3H+=Al3++3H2OΓΘ


 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ Β―ι “ΡΘΡβΚœ≥…ΝρΥαΒΡΝς≥Χ»γœ¬ΘΚ

¥”œ¬ΆΦ÷–―Γ‘ώ÷Τ»ΓΤχΧεΒΡΚœ ΉΑ÷ΟΘΚ

Θ®1Θ©ΉΑ÷ΟCΒΡΟϊ≥ΤΈΣ__________Θ§ Β―ι “Ά®≥Θ”ΟΉΑ÷ΟC÷Τ±Η_____ΓΘ
AΘ°H2 BΘ°C2H2 CΘ°CO2 DΘ°NH3
Θ®2Θ© Β―ι “”ΟΉΑ÷ΟD÷Τ±ΗO2ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________________ΓΘ
Θ®3Θ©»τ”ΟΉΑ÷ΟB÷Τ±ΗSO2Θ§Ω…“‘―Γ”Ο ‘ΦΝΈΣ_____ΓΘ
AΘ°≈®ΝρΥαΓΔ―«ΝρΥαΡΤΙΧΧε BΘ°≈®ΝρΥαΓΔΆ≠Τ§
CΘ°œΓΝρΥαΓΔ―«ΝρΥαΡΤ»ή“Κ DΘ°≈®ΝρΥαΓΔΧζ–Φ
Θ®4Θ©SO2ΚΆO2Ά®ΙΐΦΉΉΑ÷ΟΘ§ΦΉΉΑ÷ΟΒΡΉς”Ο≥ΐΝΥΩ…“‘ΩΊ÷ΤSO2ΓΔO2ΒΡΝςΥΌΆβΘ§ΜΙΩ…“‘__________ΓΔ__________ΓΘ

Θ®5Θ© Ι”Ο““¥ΠΒΦ≥ωΒΡ”–ΙΊΤχΧε÷Τ≥…ΝρΥαΘ§œ¬Ν–aΓΔbΓΔc»ΐΧΉΉΑ÷Ο÷–Ρψ―Γ‘ώΒΡ «_______Θ§ΗΟΧΉΉΑ÷Ο”κΤδΥϋΉΑ÷Ο±»ΫœΘ§Τδ”≈Βψ «___________________________________ΓΘ

≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΫπ τΦΑΤδΜ·ΚœΈοΉΣΜ·ΙΊœΒ «Μ·―ß―ßœΑΒΡ÷Ί“ΣΡΎ»ί÷°“ΜΘ§œ¬Ν–ΗςΉιΈο÷ ΒΡΉΣΜ·ΙΊœΒ≤ΜΡή»Ϊ≤ΩΆ®Ιΐ“Μ≤ΫΖ¥”ΠΆξ≥…ΒΡ «Θ® Θ©
A.NaΓζNaOHΓζNa2CO3ΓζNaClB.FeΓζFeCl3ΓζFe(OH)3ΓζFe2O3
C.MgΓζMgCl2ΓζMg(OH)2ΓζMgSO4D.AlΓζAl2O3ΓζAl(OH)3ΓζAlCl3
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“―÷ΣΘΚ¥” ·”Ά÷–ΜώΒΟA «ΡΩ«ΑΙΛ“Β…œ…ζ≤ζAΒΡ÷ς“ΣΆΨΨΕΘ§AΒΡ≤ζΝΩΆ®≥Θ”Οά¥ΚβΝΩ“ΜΗωΙζΦ“ΒΡ ·”ΆΜ·ΙΛΖΔ’ΙΥ°ΤΫΓΘœ÷“‘AΈΣ÷ς“Σ‘≠ΝœΚœ≥…““Υα““θΞΘ§ΤδΚœ≥…¬ΖœΏ»γΆΦΥυ ΨΓΘ
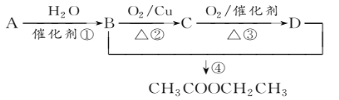
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)–¥≥ωAΒΡΫαΙΙΦρ Ϋ______________ΓΘ
(2)BΓΔDΖ÷Ή”÷–ΒΡΙΌΡήΆ≈Οϊ≥ΤΖ÷±π «_______________ΓΔ________________ΓΘ
(3)–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΖ¥”Πάύ–ΆΘΚΔΌ_________Θ§ΔΎ________Θ§Δή__________ΓΘ
(4)–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ________________________________________________________________ΘΜ
ΔΎ________________________________________________________________ΓΘ
Δέ________________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΈ“Ιζ≥« –ΈΣΝΥΦθ…Ό»ΦΟΚ‘λ≥…ΒΡ¥σΤχΈέ»ΨΘ§Ε‘”ΎΉςΟώ”Ο»ΦΝœΒΡΟΚΫχ––ΝΥΕύΖΫΟφΒΡΗΡΫχΓΘ
Θ®1Θ©ΈΣΝΥ≥ΐ»ΞΟΚ÷–ΒΡΚ§ΝρΜ·ΚœΈοΘ§≤…”ΟFeCl3Ά―ΝρΘ§Φ¥”ΟFeCl3»ή“ΚΫΰœ¥ΟΚΖέΘ§ΖΔ…ζ»γœ¬Ζ¥”ΠΘΚFeS2ΘΪ14FeCl3ΘΪ8H2O=2FeSO4ΘΪ13FeCl2ΘΪ16HClΓΘ
ΔΌΗΟΖ¥”Π÷–ΒΡ―θΜ·ΦΝ «________Θ§»τ”–1 mol FeS2±Μ≥ΐ»ΞΘ§‘ρΖΔ…ζΉΣ“ΤΒΡΒγΉ”ΒΡΈο÷ ΒΡΝΩ «________ΓΘ
ΔΎΈΣΝΥ≥δΖ÷άϊ”ΟFe2ΘΪ≤ΔΦθ…ΌΥαΘ®HClΘ©Έέ»ΨΘ§±ΨΖΫΖ®÷–Ω…άϊ”ΟΙΛ“ΒΖœΧζ–ΦΚΆ¬»Τχ»ΟΖœ“Κ÷Ί–¬άϊ”Ο…ζ≥…FeCl3ΓΘ«κ–¥≥ω’β“ΜΙΐ≥Χ÷–”–ΙΊΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_____________ΓΘ
Θ®2Θ©Νμ“Μ÷÷ΖΫΖ® «≤…”ΟΓΑΙΧΝρΖ®Γ±Θ§Φ¥‘Ύ»ΦΝœ÷–Φ”»κ…ζ ·Μ“Θ§ ΙΚ§ΝρΟΚ‘Ύ»Φ…’ ±…ζ≥…ΒΡSO2≤ΜΡή“ί≥ωΕχΫχ»κ¬·‘ϋ÷–Θ§ ‘”ΟΜ·―ßΖΫ≥Χ Ϋ±μ Ψ’β“ΜΓΑΙΧΝρΓ±Ιΐ≥ΧΘΚ_______ΓΔ________ΓΘ
Θ®3Θ©Ρ≥≥« –≤…”ΟΝΥ“‘”Ά÷ΤΤχ¥ζΧφΟΚΉςΟώ”Ο»ΦΝœΒΡΉωΖ®ΓΘ”Ά÷ΤΤχΒΡ÷ς“Σ≥…Ζ÷ «±ϊΆιΘ§«κ–¥≥ωΤδ»Φ…’ΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_____________ΓΘ
Θ®4Θ©¬Χ…ΪΡή‘¥ «»ΥάύΒΡάμœκΡή‘¥Θ§≤ΜΜα‘λ≥…ΜΖΨ≥Έέ»ΨΘ§œ¬Ν–Ρή‘¥ τ”Ύ¬Χ…ΪΡή‘¥ΒΡ «________ΓΘ
A «βΡή‘¥ B ΧΪ―τΡή C ΖγΡή D ·”Ά
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ¥”¬ΝΆΝΩσ(≥…Ζ÷ «Al2O3Θ§Κ§SiO2ΓΔFe2O3ΓΔMgOΒ»‘”÷ )÷–Χα»Γ―θΜ·¬ΝΒΡΙΛ“’Νς≥Χ»γœ¬ΘΚ
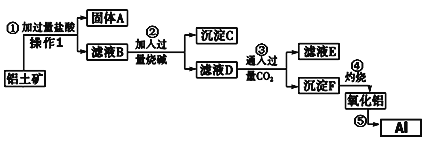
‘ρΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©≤ΌΉς1ΒΡΟϊ≥ΤΈΣ____________Θ§”ΟΒΫΒΡ≤ΘΝß“«Τς≥ΐ≤ΘΝßΑτΓΔ…’±≠ΆβΘ§ΜΙ–η_________ΘΜ
Θ®2Θ©≥ΝΒμCΒΡ≥…Ζ÷ΈΣ________________(–¥Μ·―ß Ϋ)ΘΜ
Θ®3Θ©…ηΦΤΉνΦρ Β―ι÷ΛΟς¬Υ“ΚD÷–Fe3+“―≥ΝΒμΆξ»Ϊ(–¥≥ω≤ΌΉςΓΔœ÷œσΦΑΫα¬έΘ§ ‘ΦΝ»Έ―Γ)ΘΚ»Γ2mL¬Υ“ΚD”Ύ ‘Ιή÷–Θ§__________________________ΓΘ
Θ®4Θ©¬Υ“ΚE÷–ΒΡ÷ς“Σ»ή÷ ΒΡΜ·―ß ΫΈΣ________________ΘΜ
Θ®5Θ©–¥≥ω≤Ϋ÷ηΔίΒΡΜ·―ßΖΫ≥Χ Ϋ________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΙΛ“Β…œ”Ο¬ΝΆΝΩσ(÷ς“Σ≥…Ζ÷ΈΣAl2O3Θ§Κ§”–…ΌΝΩSiO2ΓΔFeOΓΛxFe2O3Β»‘”÷ )÷Τ»Γ¬ΝΒΡ“Μ÷÷ΙΛ“’Νς≥Χ Ψ“βΆΦ»γœ¬ΘΚ
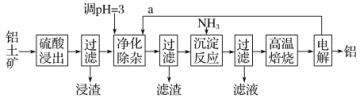
“―÷ΣΘΚ…ζ≥…«β―θΜ·Έο≥ΝΒμΒΡpH»γœ¬±μΓΘ
≥ΝΒμΈο | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
pH | 3.2 | 5.2 | 12.4 |
(1)ΈΣΧαΗΏΫΰ≥ωΥΌ¬ Θ§≥ΐ Β±‘ωΦ”ΝρΥα≈®Ε»ΆβΘ§ΜΙΩ…≤…»ΓΒΡ¥κ ©”–_______________
(2)Ϋΰ‘ϋΒΡ÷ς“Σ≥…Ζ÷ΈΣ____________________
(3)ΨΜΜ·≥ΐ‘”≤ΌΉςΖ÷ΈΣΝΫ≤ΫΘΚΒΎ“Μ≤Ϋ «Ά®»κΤχΧεaΘ§ΤδΡΩΒΡ «_____________________ΓΘΒΎΕΰ≤Ϋ «ΩΊ÷Τ»ή“ΚpHΘ§÷Μ ΙFe3+ΉΣΜ·ΈΣFe(OH)3≥ΝΒμΓΘΨΜΜ·≥ΐ‘”…ζ≥…ΒΡ≥ΝΒμ÷–ΜΙΚ§”–»ή“Κ÷–ΒΡ–ϋΗΓ‘”÷ Θ§»ή“Κ÷–ΒΡ–ϋΗΓ‘”÷ ±ΜΙ≤Ά§≥ΝΒμΒΡ‘≠“ρ «_____________________
(4)–¥≥ω≥ΝΒμΖ¥”Π≤ΌΉς÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ________________________ΓΘ
(5) Β―ι “ΗΏΈ¬±Κ…’ ±Θ§”Ο”Ύ ΔΖ≈ΙΧΧεΒΡ“«ΤςΟϊ≥Τ «_______________ΓΘ
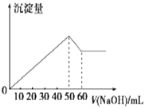
(6)‘ΎAlCl3ΚΆMgCl2ΒΡΜλΚœ“Κ÷–ΒΈΦ”NaOH»ή“ΚΘ§…ζ≥…≥ΝΒμΒΡΝΩ”κΒΈ»κNaOH»ή“ΚΒΡΧεΜΐΙΊœΒ»γΆΦΥυ ΨΘ§‘ρ‘≠»ή“Κ÷–AlCl3ΚΆMgCl2ΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ__________Θ§≤Δ–¥≥ωΦ”»κNaOH»ή“ΚΒΡΧεΜΐΈΣ50-60mL ±ΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ__________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“―÷ΣAΈΣΒ≠ΜΤ…ΪΙΧΧεΘ§TΓΔRΈΣΝΫ÷÷≥ΘΦϊΒΡ”ΟΆΨΚήΙψΒΡΫπ τΒΞ÷ Θ§D «ΨΏ”–¥≈–‘ΒΡΚΎ…ΪΨßΧεΘ§C «Έό…ΪΈόΈΕΒΡΤχΧεΘ§H «ΑΉ…Ϊ≥ΝΒμΘ§«“‘Ύ≥± ΣΩ’Τχ÷–―ΗΥΌ±δΈΣΜ“¬Χ…ΪΘ§Ήν÷’±δΈΣΚλΚ÷…ΪΙΧΧεΓΘ
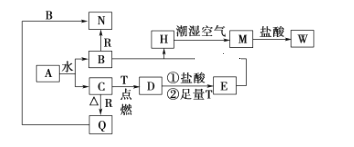
(1)–¥≥ωœ¬Ν–Έο÷ ΒΡΜ·―ß ΫAΘΚ_______________Θ§QΘΚ_____________Θ§WΘΚ__________________ΓΘ
(2)Α¥“Σ«σ–¥≥ωœ¬Ν–Ζ¥”ΠΖΫ≥Χ ΫΘΚ
ΔΌH‘Ύ≥± ΣΩ’Τχ÷–±δ≥…MΒΡΙΐ≥Χ÷–ΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_______________________
ΔΎR”κB»ή“ΚΖ¥”Π ±ΒΡάκΉ”ΖΫ≥Χ ΫΘΚ___________________________________
ΔέD”κ―ΈΥαΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_______________________________________
(3)Φρ ωΦλ―ιΤχΧεCΒΡΖΫΖ®ΘΚ___________________________________________
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «( )
A.Χλ»ΜΤχΓΔΥ°ΟΚΤχΓΔ“ΚΜ· ·”ΆΤχΨυ «…ζΜν÷–≥Θ”ΟΒΡ»ΦΝœΘ§ΥϋΟ«ΒΡ÷ς“Σ≥…Ζ÷ΕΦ «Μ·ΚœΈο
B.ΈόΥ°![]() ≥ άΕ…ΪΘ§ΈϋΥ°Μα±δΈΣΖέΚλ…ΪΘ§Ω…”Ο”Ύ≈–Εœ±δ…ΪΙηΫΚ «ΖώΈϋΥ°
≥ άΕ…ΪΘ§ΈϋΥ°Μα±δΈΣΖέΚλ…ΪΘ§Ω…”Ο”Ύ≈–Εœ±δ…ΪΙηΫΚ «ΖώΈϋΥ°
C.÷Τ≤ΘΝßΚΆΥ°ΡύΕΦ“Σ”ΟΒΫ ·Μ“ ·‘≠Νœ
D.1996Ρξ»ΥΙΛΚœ≥…ΝΥΒΎ112Κ≈‘ΣΥΊφä(Cn)Θ§ ΔΖ≈φäΒΡ»ίΤς…œ”ΠΗΟΧυΒΡ±ξ«© «![]()
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com