
(11��) ������A��B��C��D��Ϊͬ���칹�壬������Ϊ136��������ֻ��̼���⡢�����������ĺ���Ϊ23.5%��ʵ�������������A��B��C��D����һȡ�����㻯�������A��C��D�ķ���������ֻ��һ�������š�4���������ڼ��������¿��Խ������·�Ӧ��
|
10-1 д��A��B��C��D�ķ���ʽ��
10-2 ����A��B��C��D�Ľṹ��ʽ��
10-3 A��D�ֱ���NaOH��Һ���������෴Ӧ��
10-4 д��H�����й����ŵ����ơ�
10-5 ����������Һ��HCl��HNO3��NH3 • H2O��NaOH��NaHCO3������Br2ˮ��FeCl3��NH4Cl������ѡ������Լ������һ��ʵ�鷽��������E��G��I��
10-1 д��A��B��C��D�ķ���ʽ��
C8H8O2 (1��)
10-2 ����A��B��C��D�Ľṹ��ʽ��
A�Ľṹ��ʽ��
![]() ��
�� ![]() (1��)
(1��)
��д��C6H5COOCH3Ҳ�÷֡�
B�Ľṹ��ʽ��
![]() ��
�� ![]() (1��)
(1��)
��д��C6H5CH2OCHOҲ�÷֡�
C�Ľṹ��ʽ��
![]() ��
�� ![]() (1��)
(1��)
��д��C6H5OCOCH3Ҳ�÷֡�
D�Ľṹ��ʽ��
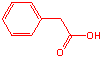 ��
�� ![]() (1��)
(1��)
��д��C6H5CH2COOHҲ�÷֡�
10-3 A��D�ֱ���NaOH��Һ���������෴Ӧ��
A��NaOH��Һ���������ļ���ˮ�ⷴӦ�� (0.5��)
�����ˮ�ⷴӦ������ˮ�ⷴӦ��ˮ�ⷴӦ��������ȡ����ӦҲ��0.5�֡�
D��NaOH��Һ����������кͷ�Ӧ�� (0.5��)
����Ӧ���кͷ�ӦҲ��0.5�֣������𰸲��÷֡�
10-4 д��H�����й����ŵ����ơ�
ȩ�����Ȼ��� ��0.5�� (��1��)
10-5 ����������Һ��HCl��HNO3��NH3 • H2O��NaOH��NaHCO3������Br2ˮ��FeCl3��NH4Cl������ѡ������Լ������һ��ʵ�鷽��������E��G��I��
����Ŀ��������Ϣ���Ƴ���E�DZ����ᣬG�DZ��״���I�DZ��ӡ����������Լ����ɲ������·���������
����1��
ȡ3���Թܣ�����2mLˮ���ֱ�����E��G��I�����Թ��У��ٸ���1-2��FeCl3 ��Һ����������ɫ���Թ��еĻ�����Ϊ����I�� (2��)
��ȡ2���Թܣ���������NaOH��Һ���ֱ�E��G�����Թ��У����������NaOH��Һ�Ļ�����Ϊ���״�G������NaOH��Һ�Ļ�����Ϊ������E��(2��)
����2��
ȡ3���Թܣ�����2mLˮ���ֱ�����E��G��I�����Թ��У��ٸ��Ӽ�����ˮ��������ɫ�������Թ��еĻ�����Ϊ����I�� (2��)
��ȡ2���Թܣ���������NaOH��Һ���ֱ�E��G�����Թ��У����������NaOH��Һ��Ϊ���״�G������NaOH��Һ��Ϊ������E�� (2��)
����3��
ȡ3���Թܣ�����2mLˮ���ֱ�����E��G��I�����Թ��У��ٸ���1-2��FeCl3 ��Һ����������ɫ���Թ��еĻ�����Ϊ����I�� (2��)
��ȡ2���Թܣ���������NaHCO3��Һ���ֱ�E��G�ӵ��Թ��С����ȣ��ų�������Թ��еĻ�����Ϊ������E�� (2��)
����4;
ȡ3���Թܣ�����2mLˮ���ֱ�����E��G��I�����Թ��У��ٸ��Ӽ�����ˮ��������ɫ�������Թ��еĻ�����Ϊ����I�� (2��)
��ȡ2���Թܣ���������NaHCO3��Һ���ֱ�E��G�����Թ��С����ȣ��ų�������Թ��еĻ�����Ϊ������E�� (2��)
����5��ȡ3���Թܣ���������NaOH��Һ���ֱ�E��G��I�ӵ��Թ��У����������NaOH��Һ�Ļ�����Ϊ���״�G������NaOH��Һ�Ļ�����Ϊ������E��I�� (2��)
��ȡ2���Թܣ���������NaHCO3��Һ���ֱ�E��I�����Թ��С����ȣ��ų�������Թ��еĻ�����Ϊ������E�� (2��)
�������5�ַ����е��κ�һ�־����֡�������������Ҳ�÷֣�������ʹ�������Լ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)������A����ÿ������ֻ��һ����ԭ�ӣ������Լ�һ�ֻ���±�ء�����A��ˮ��Ӧ��ȫˮ�������������ԭ��Ӧ�����з�Ӧ�����������ˮ����A���ˮ��Һϡ�ͺ�ֳɼ��ݣ��ֱ����һϵ��0.1mol/L���Լ����������£�
�ټ��������ữ����������������ɫ������
�ڼ����Ȼ�����Һ������������
����Һ���ữ�������������Һ����ɫ��ȥ���ټ������ᱵ��Һ��������ɫ������
���ɢ��ж���ɸû������Ԫ���У��϶����ڵ�±���� ���ɢ��ж�A��ˮ��Ӧ�����ɵ���Һ�п϶������ڵ������� ���ɢ��ж�A��ˮ��Ӧ�����ɵ���Һ�п϶����ڵ������� ��������
��Ҫȷ���û�����ķ���ʽ����ȡ11.90gA����ˮϡ����250.00mL��ȡ25.00mL��Һ���������ĸ�����غ����ᱵ��Һ��ʹ������ȫ��������ϴ�ӡ���������Ϊ2.33g����ȷ��A�Ļ�ѧʽ��д�������������̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ��ɽ���и߶���ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
(11��)��A��B��C��D��E���ֶ�����Ԫ�أ����ǵĺ˵������C��A��B��D��E��˳����������C��D���ֱܷ���A��ԭ�Ӹ�����Ϊ1��1��2��1�γɻ����CB����EA2��Ӧ����C2A����̬����EB4��E��M���������K���������2����
(1)д��A��B��EԪ�ص�����A��__________��B��_______________�� E �� ��
(2)д������ʽD2A2__________��
(3)д��D������CuSO4��Һ��Ӧ�����ӷ���ʽ_________________________
��4��C2A����__________���γɵ�________���� (����ԡ��Ǽ��ԡ�)������ԭ���ӻ������� ,�������幹���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
(11��)��A��B��C��D��E���ֶ�����Ԫ�أ����ǵĺ˵������C��A��B��D��E��˳����������C��D���ֱܷ���A��ԭ�Ӹ�����Ϊ1��1��2��1�γɻ����CB����EA2��Ӧ����C2A����̬����EB4��E��M���������K���������2����
(1)д��A��B��EԪ�ص�����A��__________��B��_______________�� E �� ��
(2)д������ʽD2A2__________��
(3)д��D������CuSO4��Һ��Ӧ�����ӷ���ʽ_________________________
��4��C2A����__________���γɵ�________���� (����ԡ��Ǽ��ԡ�)������ԭ���ӻ������� ,�������幹���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��) ������A��B��C��D��Ϊͬ���칹�壬������Ϊ136��������ֻ��̼���⡢�����������ĺ���Ϊ23.5%��ʵ�������������A��B��C��D����һȡ�����㻯�������A��C��D�ķ���������ֻ��һ�������š�4���������ڼ��������¿��Խ������·�Ӧ��
| |
(1) д��A��B��C��D�ķ���ʽ��
(2) ����A��B��C��D�Ľṹ��ʽ��
(3) A��D�ֱ���NaOH��Һ���������෴Ӧ��
(4) д��H�����й����ŵ����ơ�
(5) ����������Һ��HCl��HNO3��NH3 • H2O��NaOH��NaHCO3������Br2ˮ��FeCl3��NH4Cl������ѡ������Լ������һ��ʵ�鷽��������E��G��I��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com