
����Ŀ���л���J ���ҹ������ɹ��з���һ����ҩ�����������࣬�����г����������һ����Ԫ�����ϳ�J��һ��·��������
�ش��������⣺
��1��B�Ľṹ��ʽ��________________��C�Ľṹ��ʽ��______________��
��2��D���� E�Ļ�ѧ����ʽΪ_________________��
��3��J�Ľṹ��ʽ��________________����һ����������H�����������ɸ߷��ӻ�����Ľṹ��ʽ��_______________��
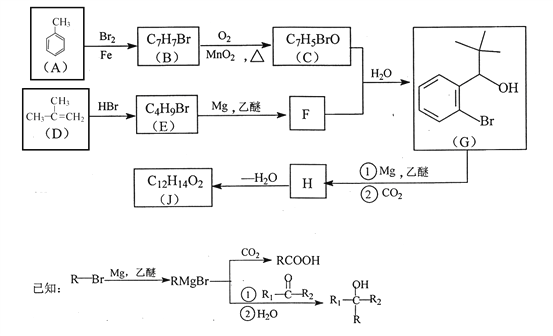
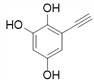
��4������![]() ��X�ķ���ʽΪ______��X�ж���ͬ���칹�壬������������������ͬ���칹�干��______�֣���֪��̼̼������̼̼˫���������ǻ�ֱ����������
��X�ķ���ʽΪ______��X�ж���ͬ���칹�壬������������������ͬ���칹�干��______�֣���֪��̼̼������̼̼˫���������ǻ�ֱ����������
A������������������������һO��O����
B������FeCl3��Һ������ɫ��Ӧ
C��������һ�ȴ���ֻ������
��5������������Ϣ����ѧ֪ʶ��д���Լ���ͼױ� Ϊԭ�����ϳ�![]() ��·������ͼ(�����Լ���ѡ)��____________________________��
��·������ͼ(�����Լ���ѡ)��____________________________��
���𰸡�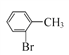

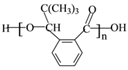 C8H6O3 9
C8H6O3 9 
��������
��1����A![]() B���������Լ�A��G�Ľṹ��ʽ��B�ķ���ʽ��֪��B�Ľṹ��ʽΪ��
B���������Լ�A��G�Ľṹ��ʽ��B�ķ���ʽ��֪��B�Ľṹ��ʽΪ��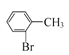 ������B
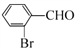
������B![]() C�����ݷ�Ӧ������C�ķ���ʽ��֪��CΪ
C�����ݷ�Ӧ������C�ķ���ʽ��֪��CΪ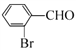 ��
��
��2��D���� E�Ļ�ѧ����ʽΪ�� ��
��
��3����G![]() H
H![]() J���Լ�������֪����֪HΪ
J���Լ�������֪����֪HΪ ������ˮ�õ�J����JΪ
������ˮ�õ�J����JΪ ����һ�������£�H�����������ɸ߷��ӻ�����Ľṹ��ʽ�ǣ�
����һ�������£�H�����������ɸ߷��ӻ�����Ľṹ��ʽ�ǣ�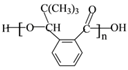 ��
��
��4������![]() ��X�Ľṹ��ʽΪ��
��X�Ľṹ��ʽΪ��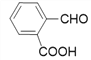 �������ʽΪ��C8H6O3��X��ͬ���칹���У�����FeCl3��Һ������ɫ��Ӧ��˵���뱽��ֱ�����������ǻ�������Ϊ�����ϵ�һ�ȴ���ֻ��һ�֣�����һO��O���������ܵ������
�������ʽΪ��C8H6O3��X��ͬ���칹���У�����FeCl3��Һ������ɫ��Ӧ��˵���뱽��ֱ�����������ǻ�������Ϊ�����ϵ�һ�ȴ���ֻ��һ�֣�����һO��O���������ܵ������


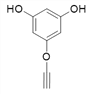
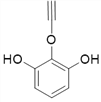


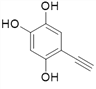
����9�֣�
��5������������Ϣ����ѧ֪ʶ��д���Լ���ͼױ� Ϊԭ�ϣ��ϳ�![]() ��·������ͼ��
��·������ͼ��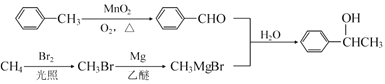 ��
��


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ijԭ�ӽṹʾ��ͼΪ �������й�˵����ȷ����(����)
�������й�˵����ȷ����(����)
A. �ṹʾ��ͼ��x��4
B. ��ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p4
C. ��ԭ�ӵĵ����Ų�ͼΪ![]()
D. ��ԭ�ӽṹ�й���5���ܼ�������е���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڸ��������£�����ѡ����ʾ�����ʼ�ת������ʵ�ֵ���
A.H2SiO3![]() SiO2
SiO2 ![]() SiCl4
SiCl4
B.Cu![]() Cu(NO3)2(aq)
Cu(NO3)2(aq) ![]() Cu(NO3)2(s)
Cu(NO3)2(s)
C.ClCH2��CH2Cl![]() HOCH2��CH2OH
HOCH2��CH2OH ![]() HOOC��COOH
HOOC��COOH
D.Al![]() Al2O3
Al2O3![]() NaAlO2(aq)
NaAlO2(aq)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���2L���ܱ������м��뷴Ӧ��N2��H2���������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
���ʵ���/ mol ʱ��/min | n(N2) | n(H2) | n(NH3) |
0 | 1.0 | 1.2 | 0 |
2 | 0.9 | ||
4 | 0.75 | ||
6 | 0.3 |
A. 0��2 min�ڣ�NH3�ķ�Ӧ����Ϊ0.1 mol��L��1��min��1
B. 2 minʱ�� H2�����ʵ���0.3 mol
C. 4 minʱ����Ӧ�Ѵﵽƽ��״̬����ʱ�����淴Ӧ�����ʶ�Ϊ0
D. 4��6 min�ڣ�������������ӵ������ʵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���ԣ�![]() ��H2CO3��
��H2CO3��![]() ����
���� ת��Ϊ
ת��Ϊ![]() �ķ����� �� ��
�ķ����� �� ��
A. ��������NaOH��Һ���ȣ���ͨ��SO2
B. ��ϡH2SO4���Ⱥ���������NaOH��Һ
C. ������Һ��ͨ��������CO2
D. ��ϡH2SO4���Ⱥ���������NaHCO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʵ���������Ҵ���Ũ���ᷴӦ������ϩ����ϩ�����巴Ӧ��1��2 -�������顣���Ʊ������в����Ҵ���Ũ��������������CO2��SO2�����������巴Ӧ����HBr���������塣
��1����������������������������Ϊԭ���Ʊ�1��2һ�������顣�����������Ϊ�����ң���ȷ������˳���ǣ��̽ӿڻ���Ƥ�ܾ�����ȥ����B��A�ٲ���A�У�D��A�ڣ�A�۽�_______��_____��_____��______��
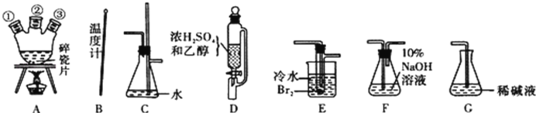
��2��װ��C��������________________��
��3���ڷ�Ӧ��E�н��е���Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________��
��ij��ȤС��ͬѧ��ʵ�����ü���l��������ŨH2SO4���廯�ƻ����ķ������Ʊ�1���嶡�飬���������ͼ��ʾ��ʵ��װ�ã����еļг�����û�л�������

��ش��������⣺
��1������װ���ж��õ��������ܣ�Aװ������ˮ��_________������ĸ���ţ����룬Bװ������ˮ��________������ĸ���ţ����롣
��2���Ʊ������У������Ũ���������������ϡ�ͣ���Ŀ����________��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3��Ϊ�˽�һ���ᴿ1���嶡�飬��С��ͬѧ�������л�����й��������±���
���� | �۵㣯�� | �е㣯�� |
1������ | ��89.5 | 117.3 |
1���嶡�� | ��112.4 | 101.6 |
���� | ��95.3 | 142.4 |
1����ϩ | ��185.3 | ��6.5 |
����Bװ����ɴ��ᴿʵ��ʱ��ʵ����ҪѸ�������¶���_________�ռ�������֡�
��4����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������________Ϊʲô����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Чɱ���¹ڲ�����2019-nCoV����ijѧϰС�������Ҫ���Ʊ�Ũ�Ȳ�С��0.8mol/L�Ĵ�������Һ��װ�ü���ͼ��
����1�����³�ѹ�£�Cl2OΪ�ػ�ɫ���壬�е�Ϊ3.8����42�����ϻ�ֽ�����Cl2��O2��Cl2O������ˮ����ˮ������Ӧ����HClO��
����2���������Ϳ���(�����뷴Ӧ)������� 1��3���ͨ�뺬ˮ8%��̼��������Cl2O������ˮ����Cl2O(����Cl2)�Ƶô�������Һ��
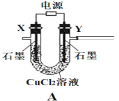
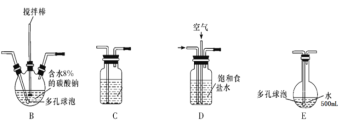

��1���缫YӦ�����ӵ�Դ��___������������������������,װ��C��Ӧʢ��_________����װ�õ�����˳��ΪA��________________________________
��2����Ӧ�����У�װ��B�������ˮ�У���Ŀ����_____________________________����֪װ��B���ﺬ��һ����ʽ�Σ�������Ӧ�Ļ�ѧ����ʽΪ___________________��
��3��ʵ���п����������������ȵķ�����_______________________________
��4��װ��E��ʹ����ɫƽ����ƿ��ԭ���ǣ��û�ѧ����ʽ��ʾ��________________
��5���˷��������������ֱ������ˮ�Ʊ���������Һ���ŵ��ǣ����һ�����ɣ�_____��
��6����װ��B�����ɵ�Cl2O������20%������Eǰ��װ���У����������װ��E��ˮ�У�װ��E����500mL��������ҺŨ��Ϊ0.8mol/L,��������Ҫ��ˮ8%��̼���Ƶ�����Ϊ___g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�����������ž�����ҵ�Ŀ��ٷ�չ�����������������Ȼ���IJ�����Ҳ��֮Ѹ����������ˣ����Ȼ���ת��Ϊ�����ļ�����Ϊ��ѧ�о����ȵ㡣�ش��������⣺Deaconֱ���������ɰ����д����̽��У�
CuCl2(s)=CuCl(s)+![]() Cl2(g) ��H1=83 kJ��mol 1
Cl2(g) ��H1=83 kJ��mol 1
CuCl(s)+![]() O2(g)=CuO(s)+
O2(g)=CuO(s)+![]() Cl2(g) ��H2= 20 kJ��mol 1
Cl2(g) ��H2= 20 kJ��mol 1
CuO(s)+2HCl(g)=CuCl2(s)+H2O(g) ��H3= 121 kJ��mol 1
��4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)����H=_________ kJ��mol 1������O2�ĵ���ʽΪ__________
(2)�����HCl��300��ʱ��Ӧ����1mol SiHCl3�����H2 ���ų�225KJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ________________________��SiHCl3�к��еĻ�ѧ������Ϊ__________
(3)��SiCl4�⻯ΪSiHCl3�����ַ�������Ӧ�ķ�Ӧ����Ϊ��
��SiCl4(g)+H2(g)![]() SiHCl3(g)+HCl(g) ��H1��0
SiHCl3(g)+HCl(g) ��H1��0
��3SiCl4(g)+2H2(g)+Si(s)![]() 4SiHCl3(g) ��H2��0
4SiHCl3(g) ��H2��0
��2SiCl4(g)+H2(g)+Si(s)+HCl(g)![]() 3SiHCl3(g) ��H3
3SiHCl3(g) ��H3
��Ӧ�۵���H3______(����H1����H2��ʾ)��
(4)����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ���������,������KClO3��H2SO4��������Na2SO3��Ӧ�Ƶ�.��д����Ӧ�����ӷ���ʽ________________________
(5)�Ȼ�麟��������ӣ��磺�ں���ͭ��ʱ���Ȼ�麟�ȥͭ�����������ͭ�Ա㺸�ӣ��䷴ӦΪ��_______CuO+______NH4Cl![]() ______Cu+______CuCl2+______N2��+______H2O
______Cu+______CuCl2+______N2��+______H2O
����ƽ��������ԭ��Ӧ����ʽ___________________________
�ڴ˷�Ӧ��������0.2mol�����壬����__________mol�ĵ���ת�ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶�����һ��2 L�ĺ����ܱ������з�����Ӧ4A(s)+3B(g)2C(g)+D(g)����2 min��ƽ��״̬����ʱB��Ӧ������0.9 mol������˵����ȷ����
A. ƽ��ʱ��v(A)��v(B)��v(C)��v(D) =4��3��2��1
B. �������ƽ����Է�����������Ϊƽ���־
C. �����������ʹѹǿ����ɼӿ췴Ӧ����
D. C��ƽ����Ӧ����Ϊ0.5 mol/(L��min)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com