
�Ժ���Al2O3��Fe2O3�����ʵĸ�����[��Ҫ�ɷ�ΪFe��CrO2��2]Ϊ��Ҫԭ�������ظ����ƾ��壨 Na2Cr2O7��2H2O������Ҫ�����������£�
��֪���������ڿ������봿����������Na2CrO4��һ�ֺ���ɫ���壬ͬʱ�ͷų�CO2���壬ͬʱA12O3+Na2CO3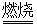 2NaAlO2+CO2������ش�
2NaAlO2+CO2������ش�
��1���ڸ�����Fe��CrO2��2�У�Cr�Ļ��ϼ�Ϊ____��
��2������1�ijɷ�Ϊ________������2�ijɷ�Ϊ____��
��3��������l����ϡ�����ܽ�����ҺW���������Һ�н������ӵķ����� ��
��4���������е�Al2O3�����ڹ�ҵ�Ͽ�����ұ�������û�ѧ����ʽΪ
��5�������йع��ұ�����CrO42���ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5��0��10��7 mo1/L���²����ŷš���CrO42���ķ�ˮ����ͨ�����������ַ�����
�ٳ���������������Ա�������BaCrO4���� ���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
�ڻ�ԭ����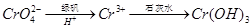 ��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
��6��ij��Ч��ˮ������K2FeO4�õ��ģ���ҵ������ҺW��������غ���������Ϊԭ���Ʊ�K2FeO4���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��1��+3��1�֣�
��2��Fe2O3 Al��OH��3����1�֣�������������Ҳ�ɣ�
��3��ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ��죬֤����Fe3+������������Ҳ�ɣ���2�֣�
��4��2 Al2O3 4Al + 3O2����1�֣�
4Al + 3O2����1�֣�
��5����2��4��10��4��2�֣���CrO42����3Fe2+��8H+��Cr3+��3Fe3+��4H2O��3�֣�
��6��Fe2��SO4��3 + 3KClO + 10KOH = 2K2FeO4 + 3K2SO4 + 3KCl + 5H2O��3�֣�
���������������1���ڸ�����Fe��CrO2��2�У�Fe�Ļ��ϼ�Ϊ+2������Cr�Ļ��ϼ�Ϊ+3��
��2�������ʵĸ��������ա�����ˮ��ֻ��������������ˮ����Ϊ������ȥ����������1�ijɷ���Fe2O3����ʱ��ҺΪNa2CrO4�� NaAlO2�Ļ��Һ��ͨ��CO2������NaAlO2��Ӧ����Al��OH��3����������2�ijɷ���Al��OH��3��
��3��������l����ϡ�����ܽ�����ҺW Fe2��SO4��3������Fe3+�ķ�����ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ��죬֤����Fe3+
��4����ҵ���õ�������������ķ������õ�������ѧ����ʽΪ2 Al2O3 4Al + 3O2����
4Al + 3O2����
��5����Ksp��BaCrO4��=1��2��10��10��c��CrO42����<=5��0��10��7 mo1/L,����c��Ba2+��>=1��2��10��10/5��0��10��7=2��4��10��4 mo1/L, ������������ CrO42�����̷�FeSO4?7 H2O��Ӧ��CrO42�������������̷�����ԭ���������ӷ���ʽΪCrO42����3Fe2+��8H+��Cr3+��3Fe3+��4H2O
��6��W ΪFe2��SO4��3������ҺFe2��SO4��3��������غ���������Ϊԭ���Ʊ�K2FeO4���÷�Ӧ�Ļ�ѧ����ʽ��Fe2��SO4��3 + 3KClO + 10KOH = 2K2FeO4 + 3K2SO4 + 3KCl + 5H2O
���㣺���黯�����л��ϼ۵��жϡ����ӵļ��顢����ұ�����ܶȻ����йؼ��㡢������ԭ��Ӧ������ж�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ������������ຬ��Cu��Au���𣩺�PbSO4�����ʣ�ʪ����������������ۺ����õ��������£�
��1����CuSO4�����Һ��⺬ͭ����Ǧ�Ĵ�ͭ�������ĵ缫��Ӧʽ�У� ��Cu-2e-= Cu2+��
��2������������ʱ��Ϊ����߱���Ч�ʣ���ȡ�ĺ�����ʩ�� �����պ���������г�����PbSO4�⣬���� ���ѧʽ����
��3������I����Ҫ����Ϊ ��������������� ��
��4��д����SO2��ԭAuCl4-�����ӷ���ʽ ��
��5��Ϊ�˼��ٷ�Һ�ŷš��������������Դ����ҵ�Ͻ���Һ1��������ͭ��Һ����ѭ����������ָ������ͼ����һ�����Ƶ����� ��
��6����֪298Kʱ��Ksp(PbCO3)=1.46��10-13��Ksp(PbSO4)= 1.82��10-8�������ӷ���ʽ��ʾ����̼������Һ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ѱ�ҵ�ĸ���ƷFeSO4����Al2(SO4)3�������ؽ������ӣ�����������ؼ��ߴ���ϸ�����������乤���������£�
��֪����5Fe2++ MnO4-+8H+=5Fe3+ +Mn2++4H2O
5C2O42-+2MnO4-+16H+=10CO2��+2Mn2++8H2O
��1���������̵ķ�Ӧ�¶�Ϊ40�棬�¶Ȳ��˹��ߵ�ԭ����˿��Ƴ����������⣬���� ��
��2����Һ�������ɵõ�����Ʒ ��
��3��ʵ���Ҳⶨ�ߴ���ϸ����������ɵIJ�������Ϊ��
����1��ȷ����һ��������������Ʒ������25 mL 2 mol��L-1��H2SO4�ܽ⡣
����2����0.2000 mol��L-1��KMnO4��Һ�ζ������������30.40 mL��
����3����ζ������Һ�м���2 g Zn�ۺ�5 mL 2 mol��L-1��H2SO4��Һ����Fe3+��ԭΪFe2+��
����4�����ˣ���Һ��������KMnO4��Һ�ζ���������Һ10.00 mL��
����Ʒ��C2O42-�����ʵ���Ϊ ����д��������̣�
��4����һ�������������Һ���ữ�IJ���������Һ��ϣ���÷�Ӧ Һ��Mn2+��Ũ���淴Ӧʱ��t�ı仯��ͼ����仯���Ƶ�ԭ�����Ϊ ��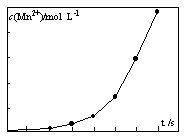
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�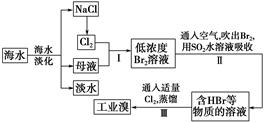
��1�����оٺ�ˮ���������ַ�����________��________��
��2����������ѻ��Br2����������ֽ�Br2��ԭΪBr������Ŀ����_________��
��3���������SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ_______��
�ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������_______��
��4��ij��ѧ�о���ѧϰС��Ϊ���˽�ӹ�ҵ�����ᴿ��ķ������������й�����֪��Br2�ķе�Ϊ59 �棬����ˮ���ж�����ǿ��ʴ�ԡ����Dzι��������̺�������װ�ü�ͼ��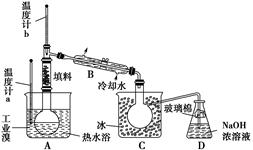
�������������ۣ�
��ͼ������B��������____________��
������ʵ��װ�����������Ӿ��������������ܣ���ԭ����__________��
��ʵ��װ�����������ã�Ҫ�ﵽ�ᴿ���Ŀ�ģ���������ο��ƹؼ�������___________��
��C��Һ����ɫΪ________________��Ϊ��ȥ�ò������Բ���������Cl2���������м���NaBr��Һ����ַ�Ӧ���ٽ��еķ��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�ִ����к�����ɳ��Ca2����Mg2����Fe3����SO�����ʡ�ijͬѧ��ʵ����������������ִ����Ʊ����εķ�������(���ڳ������Լ��Թ���)��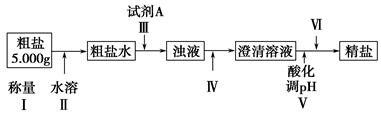
��ش��������⣺
(1)Ϊ������ѡ����������(�ñ����ĸ��д)��________��
A���ձ���B���Թܡ�C����������D����Һ©����E��©����F���ƾ��ơ�
G��������
(2)�������г���Na2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ϊ�����Լ������������Լ���˳��Ϊ��NaOH��Һ��________��________��
(3)�������У��жϼ���BaCl2�ѹ����ķ�����___________________________________
(4)������Ӧѡ�������________����������������������Ⱥ�˳��Ե��������ʵ����������Ӱ����___________________________________
(5)��������________(ѡ��������������ƣ��ñ����ĸ�������Ⱥ�˳����д)��
a�����ˡ�ϴ�� B��������Ũ�� c����ȡ����Һ D����ȴ���ᾧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���ۺ����ÿ����Ʊ�����þ������������ͼ��ʾ��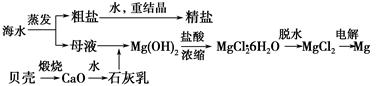
(1)Mg(OH)2�����л��е�Ca(OH)2Ӧ������ȥ��д��ʵ�鲽�衣__________________________
(2)ʵ���ҽ������Ƴɾ��εĹ����У��ܽ⡢���ˡ�������������IJ�����Ҫ�õ����������ֱ�˵���������������ʹ�ò�������Ŀ�ģ�
���ܽ⣺________________��
�ڹ��ˣ�__________________________��
��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ų�������ʯ�͡���������ˮ�������������ճ�������Ʒ����ҵ�ƹ㣬�ѽ������汻�������ӣ�����Ϊ���ִ��������͡�ս�Խ�����������߹���װ��ˮƽ���ɻ�ȱ����Ҫս�����ʡ���ҵ��Ҫ�Զ�������Ϊԭ��ұ�������ѡ�
��.�������ѿ����������ַ����Ʊ���
����1�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�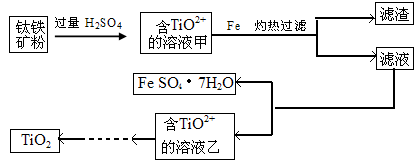
��1������Һ����̷�����IJ��������� ��
��2������Һ�г���TiO2+֮����еĽ����������� ��
��3����֪10kg������������Ԫ�ص���������Ϊ33.6%���ܹ��õ��̷�����22.24kg���Լ������ټ������۵�������
����2��TiCl4ˮ������TiO2��XH2O�����ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2���˷����Ʊ��õ��������������ѡ�
��4����TiCl4ˮ������TiO2��XH2O�Ļ�ѧ����ʽΪ ��
�ڼ���TiO2��XH2O��Cl-�Ƿ����ķ����� ��
��.�������ѿ�������ȡ�ѵ���
��5��TiO2��ȡ����Ti���漰���IJ������£�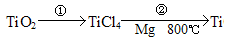
��Ӧ�ڵĻ�ѧ����ʽ�� ���÷�Ӧ�ɹ���Ҫ������������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�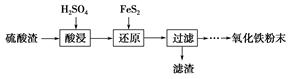
(1)�������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ���________��
(2)����ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(3)Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��ȷ��ȡһ���������������Һ����ƿ�У�����ϡ���ᡢ�Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ�Ļ�ѧ����ʽ���£�
2Fe3����Sn2����6Cl��=2Fe2����SnCl62��
Sn2����4Cl����2HgCl2=SnCl62����Hg2Cl2��
6Fe2����Cr2O72����14H��=6Fe3����2Cr3����7H2O
����SnCl2����������ⶨ��Fe3����________(�ƫ�ߡ�����ƫ�͡����䡱����ͬ)��
��������HgCl2����ⶨ��Fe3����________��
(4)�ٿ�ѡ��________(���Լ�)������Һ�к���Fe3��������Fe3����ԭ����________________________________________________________________________(�����ӷ���ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������û�������������������ų��ķ�������Ҫ��ѧ�ɷ�ΪSiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%��Ŀǰ�ҹ��Ѿ��ڼ�����ȡ��ͻ�ơ������������з�������ֳɷֲ��������á������̺�����������£�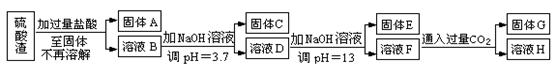
�����ϵ�֪��
| �������� | �ܶȻ�(Ksp) | pHֵ | |
| ��ʼ���� | ��ȫ���� | ||
| Mg(OH)2 | 5.6��10��12 | 9.3 | 10.8 |
| Fe(OH)3 | 2.8��10��16 | 2.7 | 3.7 |
| Al(OH)3 | 1.3��10��33 | 3.7 | 4.7 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com