
����Ŀ��CH3CH=CHCH3��������ת����ϵ��

��1���������ͬ���칹��Ľṹ��ʽΪ________��
��2��д����ͼ�з�Ӧ���ڴ����ͼ��������µĻ�ѧ����ʽ��________��
��3��CH3CH=CHCH3��ʹ��ˮ������KMnO4��Һ��ɫ��������ɫ��ԭ����ͬ��˵��ԭ��_______��
��4��ϩ��A��CH3CH=CHCH3��һ��ͬ���칹�壬���ڴ�����������������Ӧ�IJ��ﲻ�������飬��A�Ľṹ��ʽΪ___________��A�����й�ƽ���̼ԭ�Ӹ���Ϊ___________��
��5����Ӧ�ڵIJ����ͬ���칹����______�֡�
���𰸡�CH(CH3)3 CH3CH=CHCH3��H2![]() CH3CH2CH2CH3 ��ͬ��ʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ��ʹ����KMnO4��Һ��ɫ�Ƿ�����������Ӧ CH2=C(CH3)2 4 3
CH3CH2CH2CH3 ��ͬ��ʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ��ʹ����KMnO4��Һ��ɫ�Ƿ�����������Ӧ CH2=C(CH3)2 4 3
��������
��1��������CH3CH2CH2CH3����̼���칹��ͬ���칹�����춡��CH(CH3)3��
��2��CH3CH=CHCH3�������ڴ��������·����ӳɷ�Ӧ����CH3CH2CH2CH3����Ӧ����ʽ��CH3CH=CHCH3��H2![]() CH3CH2CH2CH3��
CH3CH2CH2CH3��
��3��CH3CH=CHCH3ʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ��ʹ����KMnO4��Һ��ɫ�Ƿ�����������Ӧ��������ɫ��ԭ����ͬ��
��4��CH3CH=CHCH3��ͬ���칹����CH2=CHCH2CH3��CH2=C(CH3)2��ǰ���������ӳɵIJ����������飬���AΪCH2=C(CH3)2��A�����к���̼̼˫������̼̼˫���е�̼ԭ��ֱ��������ԭ�Ӽ�̼̼˫���е�����̼ԭ�ӣ���6��ԭ�ӿ϶���ƽ�棬��A�����й�ƽ���̼ԭ�Ӹ���Ϊ4��
��5����Ӧ���IJ����ͬ���칹����CH3CH2CH2CH2Br��(CH3)2CHCH2Br��(CH3)3CBr����3�֡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ҵ�����У����뷴Ӧ���Ļ��������NO��O2�����ʵ��������ֱ�Ϊ0.10��0.06��������ѧ��Ӧ2NO(g)+O2(g)=2NO2(g)��������������ͬʱ�����ʵ���������±���
ѹǿ/(��105Pa) | �¶�/�� | NO�ﵽ����ת������Ҫʱ��/s | ||
50% | 90% | 98% | ||
1.0 | 30 | 12 | 250 | 2830 |
90 | 25 | 510 | 5760 | |
8.0 | 30 | 0.2 | 3.9 | 36 |
90 | 0.6 | 7.9 | 74 | |
���ݱ������ݣ�����˵����ȷ����
A. �����¶ȣ���Ӧ���ʼӿ�
B. ����ѹǿ����Ӧ���ʱ���
C. ��1.0��105Pa��90�������£���ת����Ϊ98%ʱ�ķ�Ӧ�Ѵﵽƽ��
D. �����뷴Ӧ���Ļ������Ϊamol����Ӧ������v=��n/��t��ʾ������8.0��105Pa��30��������ת���ʴ�50%����90%ʱ��NO�ķ�Ӧ����Ϊ4a/370mol/s
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��þ���Ͻ�Ͷ�뵽1mol/L��������Ͻ���ȫ�ܽ������Һ�����1mol/L NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ����仯�Ĺ�ϵ��ͼA��ʾ������˵���в���ȷ���ǣ� ��

A.a��ȡֵ��ΧΪ0��a��50
B.![]() �����ֵΪ2.5
�����ֵΪ2.5
C.������ϵͼ��ΪBͼʱ����a��ȡֵ��ΧΪ80��a��90
D.������ϵͼ��ΪCͼʱ����a��ȡֵ��ΧΪ75��a��90
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ľṹ����ͼ��ʾ����֪������ṹ��Ԫ������ԭ����ɵ�����ʮ���壬������20���ȱ������ε����һ����Ŀ�Ķ��㣬ÿ������ϸ���1��Bԭ�ӡ������й�˵������ȷ����

A.ÿ������Ӻ���12����ԭ��
B.�������ǿռ���״�ṹ
C.�����������60��
D.ÿ������Ӻ���30������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������(Na2S2O4)�׳Ʊ��շۣ��㷺���ڷ�֯��ҵ�Ļ�ԭ��Ⱦɫ����ϴ��ӡ������ɫ�Լ�֯���Ư�ȡ���ȡ���շ�ͨ����Ҫ��������
��1���Ʊ���������

��ʹ����ͼ��ʾװ���Ʊ������SO2���壬�ش��������⣺
��A��ʢҺ��IJ�������������____________��ʵ�鿪ʼ��A�з�Ӧ�Ļ�ѧ����ʽΪ______________________________________________________��
��Bװ�õ�������_______________________��Cװ�õ�����____________________��
��E�й����Լ�Ϊ________________��
��2���Ʊ����շ�
����ͼ��ά��35��45��ͨSO2��п��-ˮ����Һ��Ӧ����ZnS2O4��Ȼ�����18%������������Һ����28��35���·�Ӧ����Na2S2O4��Zn(OH)2����Һ��

��Ӧ�ᆳѹ�˳�ȥ������п����������Һ�м����Ȼ��ƣ�����ȴ��20�棬ʹNa2S2O4�ᾧ�������˳�������þƾ���ˮ���T�ò�Ʒ��
������ȡNa2S2O4�����з�����������ԭ��Ӧ����������___________������1mol Na2S2O4ת�Ƶ���______mol��
����Һ�м����Ȼ���ʹ_______����Ũ�����ٽ�Na2S2O4�ᾧ�������˳�������þƾ���ˮ��������ΪNa2S2O4�ھƾ��е��ܽ��_______����ϴ�С�������Ҿƾ��ӷ���
����ȡNa2S2O4Ҳ���ü����Ʒ��������¶�70��80�����ڼ״���Һ���ܼ������ܽ�����ƣ�HCOONa�����ٵμ�Na2CO3��ҺͬʱͨSO2ά����Һ����,��������Na2S2O4����Ӧ�����ӷ���ʽ��_________________________________________��
��3���ⶨ���շ۴���
Na2S2O4����ǿ��ԭ������¶�ڿ������ױ�����������Na2S2O4��KMnO4������Һ������Ӧ��5Na2S2O4 + 6KMnO4 + 4H2SO4 = 5Na2SO4 + 3K2SO4 + 6MnSO4 + 4H2O
��ȡ3.0g Na2S2O4��Ʒ������ˮ�У����100mL��Һ��ȡ��10mL����Һ���Թ��У���0.10mol��L-1��KMnO4��Һ�ζ���
�ظ���������2�Σ�ƽ������KMnO4��Һ18.00 mL�����Ʒ��Na2S2O4����������Ϊ_____________�����ʲ����뷴Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���뼰�仯�����Ӧ�������汻���ӡ�
(1)����Ҫ�ĺ������������ʯ����2%��(Cr)������ʯ��Ϊ��ĸ�̡���̬Crԭ�Ӽ۵��ӵĹ����ʾʽΪ__________��
(2)���������������Ԫ���������ơ������й��������������ȷ����______(����ĸ)��
A��������p������Ԫ�� B���縺�Զ���þ��
C����һ�����ܶ���þ�� D���Ȼ����ˮ��ҺpH��С��7
(3)�Ȼ�������̬ʱ����BeCl2����(a)�Ͷ��۷���(BeCl2)2(b)����̬ʱ���������ͼ��ʾ����״�ṹ(c)��

��a����________(���������������Ǽ�����)���ӡ�
�ڶ��۷���(BeCl2)2��Beԭ�ӵ��ӻ���ʽ��ͬ��������ԭ�Ӷ���ͬһƽ���ϡ�b �ĽṹʽΪ__________(�����λ��)��
(4)BeO������������ͼ��ʾ����BeO������ܶ�Ϊd g��cm-3��������a=______ nm��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йظ����ж�����������ȷ���ǣ� ��
A.1mol H2ȼ�շų�������ΪH2��ȼ����
B.Na2SO3��H2O2�ķ�ӦΪ������ԭ��Ӧ
C.![]() ��
��![]() ��Ϊͬϵ��
��Ϊͬϵ��
D.BaSO4��ˮ��Һ�����磬��BaSO4���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������ʯ�����ڳ������Ƭ�������²���C2H4������C2H4���ʵ�ʵ�飬������и����⡣
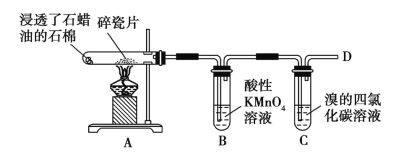
��B����Һ��ɫ������Ϊ��ϩ��______________��
��C�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
����D����ȼʱ������еIJ�����_____________��
��2��ʵ������ȡ����ϩ�г�����������SO2�����������ͼʵ��װ����֤��������������к�����ϩ�Ͷ��������Իش���������:
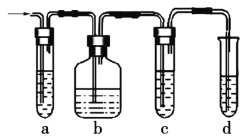
��ͼ��a��b��c��dװ��ʢ�ŵ��Լ�������___(�����)��
A��Ʒ����Һ
B��NaOH��Һ
C��Ũ����
D�����Ը��������Һ
����˵��SO2���ڵ�ʵ��������_____________��
��ʹ��װ��b��Ŀ����_____________��
��ʹ��װ��c��Ŀ����_____________��
����֤����������к�����ϩ��������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������棨CeO2����һ����Ҫ��ϡ�������ƽ�������ʾ�����������в��������ķϲ�����ĩ����SiO2��Fe2O3��CeO2�Լ���������������ϡ������ʣ���ij�������Դ˷�ĩΪԭ�ϻ����棬���ʵ���������£�
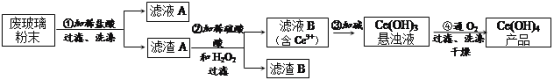
��1��ϴ������A��Ŀ����Ϊ��ȥ��_______�������ӷ��ţ�������������Ƿ�ϴ�ӵķ�����_________________________________________________________��
��2����������Ӧ�����ӷ���ʽ��______________________________������B����Ҫ�ɷ���_________��
��3����ȡ�Ƿ���ϡ��Ԫ�صij��÷�������֪������TBP��Ϊ��ȡ���ܽ������Ӵ�ˮ��Һ����ȡ������TBP________������������������������ˮ���ܡ�ʵ���ҽ�����ȡ�������õ�����Ҫ����������_________���ձ�������������Ͳ�ȡ�
��4��ȡ���������еõ���Ce(OH)4��Ʒ0.536g���������ܽ����0.1000molL-1FeSO4����Һ�ζ��յ��ǣ��汻��ԭΪCe3+��������25.00mL����Һ���ò�Ʒ��Ce(OH)4����������Ϊ__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com