
�������������ʵ�����Ʒ����������������ա�
![]() ��1���������շ�������ȡ�������壬����ȡ�����ԭ�Ͽ�ѡ��________��
��1���������շ�������ȡ�������壬����ȡ�����ԭ�Ͽ�ѡ��________��
![]() a��ϡ������������ b��ϡ������������
a��ϡ������������ b��ϡ������������
![]() c��ϡ���������� d��ϡ������������
c��ϡ���������� d��ϡ������������
![]() ��2����Ҫ��װһ���Կ�����������������ʵ�װ�ã�������ͼ��ѡ����ʵ�������
��2����Ҫ��װһ���Կ�����������������ʵ�װ�ã�������ͼ��ѡ����ʵ�������
![]()
 ______________�����ţ���
______________�����ţ���
![]()
![]()

![]()
![]()
![]()
![]() ��3������ͼ����һ�����������ļ���ƿ�м�������Ʒ��ϡ����Һ��
��3������ͼ����һ�����������ļ���ƿ�м�������Ʒ��ϡ����Һ��
![]() ��ȼ�������塣�ڻ����Զ�Ϩ���ֹͣͨ�����壬ƿ�ڿɹ۲쵽
��ȼ�������塣�ڻ����Զ�Ϩ���ֹͣͨ�����壬ƿ�ڿɹ۲쵽
![]() ��������___________________________________________________��
��������___________________________________________________��
![]() ��4������������ƿ�м���ͨ���������壬��������Ӧ�Ļ�ѧ����ʽΪ��
��4������������ƿ�м���ͨ���������壬��������Ӧ�Ļ�ѧ����ʽΪ��
![]() ______________________________________________________________
______________________________________________________________
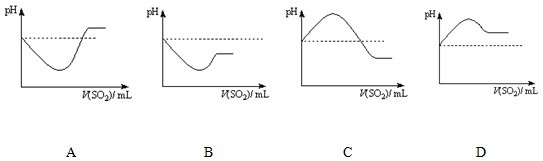
![]() ��Ӧ�����У���Һ��pH______����������С�����䡱����
��Ӧ�����У���Һ��pH______����������С�����䡱����
![]() ��5����ȼ����������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��
��5����ȼ����������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��
![]()

![]() ��6����֪���������ڿ����е��������Ϊ4.3%��45.5%ʱ�ᷢ����ը�������������ڿ����е��������Ϊ30%ʱ���䱬ը������______________________��
��6����֪���������ڿ����е��������Ϊ4.3%��45.5%ʱ�ᷢ����ը�������������ڿ����е��������Ϊ30%ʱ���䱬ը������______________________��
![]()
![]()
�𰸣�
![]() ��1��a d
��1��a d
![]() ��2���� �� ��
��2���� �� ��
![]() ��3��Ʒ����Һ��ɫ��ƿ���е���ɫ��ĩ����ɫ��СҺ��
��3��Ʒ����Һ��ɫ��ƿ���е���ɫ��ĩ����ɫ��СҺ��
![]() ��4��
��4��![]() ���
���
![]() ��5��b
��5��b
![]() ��6��S H2O
��6��S H2O
����������1���������շ�������ȡ���壬��ѡ�Լ�ӦΪ��״�����Һ�壬�ҷ�Ӧ����Ҫ���ȣ���a��d��ȷ��c�����������ǿ�����ԣ�����������Ӧ�ò���H2S��b�����
��2��Ҫ������������������ʱ���ѡ�÷�Һ©����
��3����ʼ�������㣬H2Sȼ������SO2��H2O�������������㣬ȼ�ղ���ΪS��H2O��������Ӧ����Ҫ��SO2��S��H2O�������濼�ǡ�
��5��a�����ӵ�װ�����κ����á�bװ�ÿɽ����շ������ڵ�H2S���ȼ��H2S�ָ������ܷ�ֹ��ը��c��dװ�������ɵ�H2S���ų����ʴ���
��6���������ڿ����е��������Ϊ30%ʱ��O2�ڻ�������е��������Ϊ70%��![]() =14%��O2���㣬������Ӧ2H2S��O2
=14%��O2���㣬������Ӧ2H2S��O2![]() 2S��2H2O��
2S��2H2O��
![]()
![]()
![]()


 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���ϣ���H2S������ˮ��Լ1��2������ˮ��ҺΪ��Ԫ���ᣮ ��H2S��������������ӷ�Ӧ���ɳ����� ��H2S�ڿ�����ȼ�գ�����ʵ���ɫ�� |
| ʵ����� | ʵ������ | |
| ʵ��1 | ����Ũ�ȵ�Na2S��Na2SO3��Һ�������2��1��� | ���������� |
| ʵ��2 | ��H2Sͨ��Na2SO3��Һ�� | δ�����Գ������ټ�������ϡ���ᣬ������������dz��ɫ���� |
| ʵ��3 | ��SO2ͨ��Na2S��Һ�� | ��dz��ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 0.085cV |
| �� |
| 0.085cV |
| �� |
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ̽�������� | ȡ��������������Һ���Թ��У����� ����������Һ����ȡ�����⻯�ص�����Һ���Թ��У��������������Һ���� ȡ��������������Һ���Թ��У����� ����������Һ����ȡ�����⻯�ص�����Һ���Թ��У��������������Һ���� |
��������ɫ��������Һ����ǣ�����Һ����ɫ���� ��������ɫ��������Һ����ǣ�����Һ����ɫ���� |
| ��̽�����ȶ��� | ȡ��������������Һ���Թ��У����ȣ� �ô����ǵ�ľ�����飮��ȡ��������������Һ���Թ��У����ȣ��õ����ܽ��õ�������ͨ�뵽װ�б���������Һ���Թ��У��� ȡ��������������Һ���Թ��У����ȣ� �ô����ǵ�ľ�����飮��ȡ��������������Һ���Թ��У����ȣ��õ����ܽ��õ�������ͨ�뵽װ�б���������Һ���Թ��У��� |
�������ݣ�ľ����ȼ������Һ����ǻ���dz��ɫ���������� �������ݣ�ľ����ȼ������Һ����ǻ���dz��ɫ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��־��ϵ�б���һѵ����ѧ�ս̰� �ս̰� ���ͣ�058
��֪����FeS���ܽ���ˮ����ʵ������ȡH2S��SO2�����ԭ����FeS��H2SO4![]() FeSO4��H2S����Na2SO3��H2SO4
FeSO4��H2S����Na2SO3��H2SO4![]() Na2SO4��H2O��SO2����������������������������ᷢ����Ӧ��2H2S��SO2
Na2SO4��H2O��SO2����������������������������ᷢ����Ӧ��2H2S��SO2![]() 3S����2H2O��
3S����2H2O��
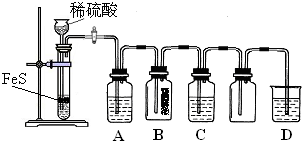
������ͼ�е�ʵ��װ�ú��Լ�����ʵ�飬����������⣺

(1)װ��A����˫�������������ܼ�________��________��װ�����ģ�
(2)��װһ����ȡSO2�����װ�ã���֤��SO2���������ԣ����л�ԭ�Ժ�Ư���ԣ�
�ٰ�ʵ��װ������˳��a��f�����ܿ�������������ȷ��˳����________��
A��befcda
B��adcefb
C��acdfeb
D��acdefb
����Cװ���е���ҺΪ________����Ӧ�����Һ��Ϊ��ɫ��˵��SO2���л�ԭ�ԣ�
����Cװ���е���ҺΪ________����Ӧ�����Һ��Ϊ��ɫ��˵��SO2����Ư���ԣ�
�ܵ�D�в���________����ʱ��˵��SO2���������ԣ�
(3)Dװ���ձ���NaOH��Һ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08���Ϻ�����![]() ���ѧ��ʵ��Ҫע�ⰲȫ������Ⱦ���������ԡ��������շ�����ԭ�������õײ���С���Թ��Ƽ������巢����(����ͼ)�����ر�K������ʹ��Ӧֹͣ���ɽ��Թܴ��ձ���ȡ��(���в���������ɢ)�������������ȡ��ʹ�ø�װ�õ��ǣ� ��
���ѧ��ʵ��Ҫע�ⰲȫ������Ⱦ���������ԡ��������շ�����ԭ�������õײ���С���Թ��Ƽ������巢����(����ͼ)�����ر�K������ʹ��Ӧֹͣ���ɽ��Թܴ��ձ���ȡ��(���в���������ɢ)�������������ȡ��ʹ�ø�װ�õ��ǣ� ��
A���ö�������(��ĩ)��˫��ˮ������ B����п����ϡ����������
C����������(��״)������������ D����̼���(��״)��ϡ�����ƶ�����̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)ʵ������ȡ����Ļ�ѧ����ʽ____________________________________��
(2)����װ��ͼ����������⡣

����ȡ��������ķ���װ�ÿ���ѡ��_____________��
������Cװ���ռ��������壬������ӦΪ_____________��ԭ����___________________��
��Ϊ����֤�����ˮ��Һ�����ԣ����Խ�����ͨ��װ��D��D����ʢ���Լ�Ӧ����_____________��������_______________________��
��������ʵ��ʱ��Ϊ�˷�ֹ����������ݳ���Ⱦ���������Խ���ͨ��װ��E���գ�E��©��������Ϊ____________________________��
������װ�õ����ӿ�����˳��Ϊ(��дa��b��c�ȷ���)____________________��
(3)����
���ռ�����H2S��������(��״��)��
����Һ��Fe2+��H+�����ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com