

(1)X�Ļ�ѧʽΪ__________________��O�Ľṹ��ʽΪ__________________��
(2)���ǵ��ۺϾ���Ч�棬��ҵ����Mʱ���ɲ�ȡ�Ĵ�ʩ��__________________(�����)��
a.ʹ�ô��� b.�ʵ������¶�
c.ѡ����ʵ��¶� d.�ʶ�����ѹǿ
e.��ʱ��������� f.��ԭ�Ͻ���ѭ������
g.������÷�Ӧ�����ų������� h.������ַ������ѡ���ڽ�ͨ����ij���
i.������ַ��ѡ����ʢ����Ȼ���ĵ���
(3)�豸I������__________����ҵ������Ϊ�˽�Լ��Դ�����ͳɱ��ͱ���������������ijЩ���ʽ���ѭ�����á�����ת����ϵ���ܴﵽ��Ŀ�ĵ���__________(�����)��
(4)����1 mol A�μӷ�Ӧ�������������ȫ��Ӧ������N������Ϊ
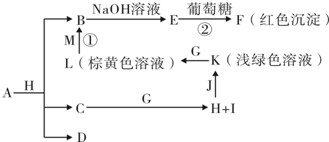
��������(1)�����ͻ�ƿ��ǡ�FΪ�����ĺ���ɫ�����������ϵ���Ҫ�ɷ֡���F������Fe2O3�����ݡ�J+Ba(NO3)2��Һ![]() ��ɫ����N����֪��N��BaSO4���ó�A�����к�����Ԫ�غ���Ԫ�أ�A���������������E��SO2��X��˫��ˮ(H2O2)��J��H2SO4����ΪB�Ƿǽ������ʣ�����B������(O2)����C(�ǽ�������)+D(������)
��ɫ����N����֪��N��BaSO4���ó�A�����к�����Ԫ�غ���Ԫ�أ�A���������������E��SO2��X��˫��ˮ(H2O2)��J��H2SO4����ΪB�Ƿǽ������ʣ�����B������(O2)����C(�ǽ�������)+D(������)![]() G(������)+H(�ǽ�������)������������C+H2O
G(������)+H(�ǽ�������)������������C+H2O![]() CO+H2��2C+SiO2
CO+H2��2C+SiO2![]() 2CO+Si����Ҫ���û���Ӧ���з�����֤��D����������ת��ͼ�г��������Σ���O�dz������ʡ����ɴ˷�ɢ���룬�����ĵ�����̼�����(̼�)�������(���)�������(���)���Ȼ�狀����صȣ���ϡ���Ӧ����L��M�����ʵ���֮��Ϊ1��
2CO+Si����Ҫ���û���Ӧ���з�����֤��D����������ת��ͼ�г��������Σ���O�dz������ʡ����ɴ˷�ɢ���룬�����ĵ�����̼�����(̼�)�������(���)�������(���)���Ȼ�狀����صȣ���ϡ���Ӧ����L��M�����ʵ���֮��Ϊ1��![]() CO(NH2)2+H2O����һ���ó�C������̼��G��CO��H��������I��N2��
CO(NH2)2+H2O����һ���ó�C������̼��G��CO��H��������I��N2��
(2)��ҵ������NH3����������ý����������ѡ��![]() CO2+4H2)��
CO2+4H2)��
(3)�豸���Ǻϳ������豸������ȴ�豸�ͷ�����������N2��H2��ѭ�����ù��̡�
(4)n(Fe)=![]() =4 mol��n(BaSO4)=
=4 mol��n(BaSO4)=![]() =5 mol��˵��������A����������Ԫ�ص�ԭ�Ӹ���֮��Ϊ4��5����ѧʽΪFe4S5�����Է�Ӧ�ٵĻ�ѧ����ʽΪFe4S5+8O2
=5 mol��˵��������A����������Ԫ�ص�ԭ�Ӹ���֮��Ϊ4��5����ѧʽΪFe4S5�����Է�Ӧ�ٵĻ�ѧ����ʽΪFe4S5+8O2![]() 2Fe2O3+5SO2��
2Fe2O3+5SO2��
���𰸡�(1)H2O2 ![]() (2)acdefgi
(2)acdefgi
(3)�ϳ��� �� (4)Fe4S5+8O2![]() 2Fe2O3+5SO2
2Fe2O3+5SO2


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
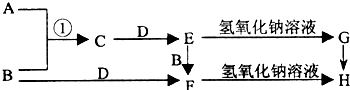
 ��2012?����һģ����֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��������D��GΪ��ɫ��ζ���壮��֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ��ͼ�����ַ�Ӧ��������ȥ����
��2012?����һģ����֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��������D��GΪ��ɫ��ζ���壮��֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ��ͼ�����ַ�Ӧ��������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com