
�����г����۵�ij��ʳ�þ����ΰ�װ����������˵����
| ��Ʒ�� | GB5461 |
| ��Ʒ�ȼ� | һ�� |
| ���� | ʳ�Ρ�����ء������ |
| �⺬������I�ƣ� | 20��50mg/kg |
| ��װ���� | |
| ��װ��ҵ |
��1���������⻯�������������·������·�Ӧ����ƽ��ѧ����ʽ������ѧ���������ڿհ״�����
___KIO3+_____KI+_____H2SO4====_____K2SO4+_____I2+______H2O
��2��������Ӧ���ɵ�I2�������Ȼ�̼���顣�������Ȼ�̼��Һ�м���Na2SO3ϡ��Һ����I2��ԭ���Ի������Ȼ�̼��
��Na2SO3ϡ��Һ��I2��Ӧ�����ӷ���ʽ��_______________________________________��
��ijѧ����ƻ������Ȼ�̼�IJ�������Ϊ��
a.��������Ȼ�̼��Һ���ڷ�Һ©���У�
b.��������Na2SO3ϡ��Һ��
c.������²�Һ�塣
�����������©�IJ����������������е�λ����______________________________��
��3����֪��I2+2 ====2I-+
====2I-+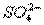 ��
��
ijѧ���ⶨʳ�þ����εĵ⺬�����䲽��Ϊ��
a.ȷ��ȡw gʳ�Σ�����������ˮʹ����ȫ�ܽ⣻
b.��ϡ�����ữ������Һ����������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��
c.�Ե�����ҺΪָʾ������μ������ʵ���Ũ��Ϊ2.0��10-3 mol/L��Na2S2O3��Һ10.0 mL��ǡ�÷�Ӧ��ȫ��
���ж�c�з�Ӧǡ����ȫ���ݵ�������______________________________��
��b�з�Ӧ��������I2�����ʵ�����______________________________mol��
�۸�������ʵ��Ͱ�װ��˵�������⾫���εĵ⺬���ǣ��Ժ�w�Ĵ���ʽ��ʾ��
______________________________mg/kg��
���𰸡�
��1��1��5��3��3��3��3
��2����I2+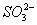 +H2O====2I-+
+H2O====2I-+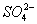 +2H+
+2H+
���ڲ���b�����Ӳ���������Һ©���������
��3������Һ����ɫǡ�ñ�Ϊ��ɫ
��1.0��10-5
��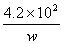
���������� ����Ҫ����ⶨʳ���е�ĺ�����ʵ���⡣�û��ϼ�����������ƽ��ѧ����ʽ��
����Ҫ����ⶨʳ���е�ĺ�����ʵ���⡣�û��ϼ�����������ƽ��ѧ����ʽ��
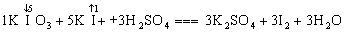
Na2SO3��I2��Ӧ��SO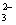 +I2+H2O===SO
+I2+H2O===SO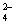 +2I��+2H+
+2I��+2H+
��ΪNaI��Na2SO4������ˮ��CCl4������ˮ���÷�Һ���ֿ�����������������©�IJ�������b����֮�����ӽ���Һ©��������á���ΪI2+2S2O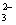 ===2I��+S4O
===2I��+S4O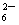 ǡ����ȫ��Ӧ��I2�������꣬��Һ��ɫǡ�ñ����ɫ��
ǡ����ȫ��Ӧ��I2�������꣬��Һ��ɫǡ�ñ����ɫ��
I2+2S2O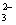 ===2I��+S4O
===2I��+S4O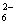
n(I2)=2.0��10��3 mol��L��1��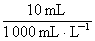 ��
��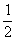 =1.0��10��5 mol
=1.0��10��5 mol
5KI+1KIO3+3H2SO4===3K2SO4+3I2+3H2O
n(KIO3)= ��1.0��10��5 mol
��1.0��10��5 mol
����ʳ���е⺬��
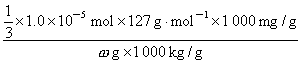 =
=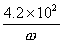 mg/kg
mg/kg



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������NOx��NO��NO2�Ļ����(����N2O4)��
(1)���ݷ����ŷű���1 m3�������������400 mg NOx����NOx��NO��������Ϊ0.85����1 m3���������������NO___________________L(��״��������2λС��)��
(2)��ҵ��ͨ����������������Ϊ0.150��Na2CO3ˮ��Һ(�ܶ�1.16 g��mL-1)��ΪNOx���ռ�����̼������Һ���ʵ���Ũ��Ϊ__________________________mol��L-1(����2λС��)��
(3)��֪��NO+NO2+Na2CO3 2NaNO2+CO2��
2NaNO2+CO2��
2NO2+Na2CO3 NaNO2+NaNO3+CO2��
NaNO2+NaNO3+CO2��
1 m3��2 000 mg NOx����������������Ϊ0.150��̼������Һ���ա���������Ϊ80%�����պ������_______________�ŷű�(����ϡ������ϡ�)�����ɣ�_________________��
(4)��������ɸı�������NO��NO2�ıȣ���ӦΪ��NO+2HNO3 3NO2+H2O
3NO2+H2O
��������n(NO)��n(NO2)=2��3ʱ����������ߡ�
1 m3������2 000 mg NOx������n(NO)��n(NO2)=9��1��
���㣺(��)Ϊ�˴ﵽ��������ʣ�1 m3����������������ʵ���(����3λС��)��
(��)1 m3�����ﵽ���������90%ʱ�����պ�����NaNO2������(�����������շ�Ӧ�У���Ӧ�ٱȷ�Ӧ��Ѹ�١�����������1λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.Ŀǰ�����ڶ�����̼�Ƿ�Ϊ������Ⱦ���в�ͬ�Ĺ۵㡣��Ϊ��������̼���Ǵ�����Ⱦ���������( )
�ٶ�����̼����Ҫ�Ļ���ԭ��
�ڶ�����̼��ֲ�������õı���ԭ��
�۶�����̼����ɫ����ζ����������
�ܳ�������̼�⣬���顢һ��������Ҳ����������
A.�٢� B.�ڢ� C.�ۢ� D.�٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
U��V��W��X��Y��Z��ԭ������������������ֳ���Ԫ�ء�Y�ĵ�����W2��ȼ�յIJ����ʹƷ����Һ��ɫ��Z��WԪ���γɵĻ�����Z3W4���д��ԡ�U�ĵ�����W2��ȼ�տ�����UW��UW2�������塣X�ĵ�����һ�ֽ������ý�����UW2�о���ȼ�����ɺڡ������ֹ��塣
��ش��������⣺
��1��V�ĵ��ʷ��ӵĽṹʽΪ ��XW�ĵ���ʽΪ ��ZԪ�������ڱ��е�λ���� ��
��2��UԪ���γɵ�ͬ��������ľ������Ϳ����ǣ�����ţ� ��
��ԭ�Ӿ��� �����Ӿ��� �۷��Ӿ��� �ܽ�������
��3��U��V��W�γɵ�10�����⻯���У�U��V���⻯��е�ϵ͵��ǣ�д��ѧʽ��
��V��W���⻯����ӽ��H+������ǿ���ǣ�д��ѧʽ�� ����һ�����ӷ���ʽ����֤�� ��
��4��YW2����ͨ��BaCl2��HNO3�Ļ����Һ�����ɰ�ɫ��������ɫ����VW���йط�Ӧ�����ӷ���ʽΪ ���ɴ˿�֪VW��YW2��ԭ�Խ�ǿ���ǣ�д��ѧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ����������Ϊԭ����������������β�����˺���N2��O2�⣬������SO2������SO3��������Ϊ�˱���������ͬʱ������Ṥҵ���ۺϾ���Ч�棬Ӧ�����ܽ�β���е�SO2ת��Ϊ���õĸ���Ʒ���밴Ҫ��ش��������⣺
��1����β��ͨ�백ˮ�У��ܷ��������Ӧ��д�����п��ܷ���������������ԭ��Ӧ�Ļ�ѧ����ʽ�� �� ��
��2����β���백ˮ��Ӧ���õ��ĸ�Ũ����Һ�У���һ���������백ˮ��̼����泥���ʱ��Һ���¶Ȼ����н��ͣ����� �����塣�ٵ�����Һ�¶Ƚ��͵�ԭ������� ;�������ľ����������ֽ��ҵ��Ҳ��������������ӰҺ����������֪�ýᾧˮ�������Է�������Ϊ134�����仯ѧʽΪ ����������������Ҫ����Һ�м��������ĶԱ����ӻ�Ա����������ʣ���Ŀ���� ��
�����塣�ٵ�����Һ�¶Ƚ��͵�ԭ������� ;�������ľ����������ֽ��ҵ��Ҳ��������������ӰҺ����������֪�ýᾧˮ�������Է�������Ϊ134�����仯ѧʽΪ ����������������Ҫ����Һ�м��������ĶԱ����ӻ�Ա����������ʣ���Ŀ���� ��
��3�������ڲⶨ����β����SO2�������� ��������ĸ��
A.NaOH��Һ����̪��Һ B.KMnO4��Һ��ϡH2SO4
C.��ˮ��������Һ D.��ˮ����̪��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ������ղ���ΪSO2��Fe3O4��
��1����0.050molSO2(g)��0.030molO2(g)�����ݻ�Ϊ1L���ܱ������У���Ӧ��2SO2(g)+O2(g)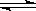 2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol/L������������·�Ӧ��ƽ�ⳣ��K��SO2��ƽ��ת���ʣ�д��������̣���
2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol/L������������·�Ӧ��ƽ�ⳣ��K��SO2��ƽ��ת���ʣ�д��������̣���
��2����֪������Ӧ�Ƿ��ȷ�Ӧ�����÷�Ӧ����ƽ��״̬ʱ�����������������£����д�ʩ�����������SO2ƽ��ת���ʵ��� ������ĸ��
A �����¶� B �����¶� C ����ѹǿ
D ��Сѹǿ E ������� G �Ƴ�����
��3��SO2β���ñ���Na2SO3��Һ���տɵõ���Ҫ�Ļ���ԭ�ϣ���Ӧ�Ļ�ѧ����ʽΪ
��
��4��������������ղ���Fe3O4����H2SO4�������ۣ����Ʊ�FeSO4�����ܹ������豣����Һ�㹻���ԣ���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�Ļ�ѧ����ʽ��
A.���������գ�2FeS2+5O2 2FeO+4SO2
2FeO+4SO2
B.ʯӢ��ʯ��ʯ���ۣ�SiO2+CaO CaSiO3
CaSiO3
C.���Ĵ�������4NH3+5O2 4NO+6H2O
4NO+6H2O
D.������ʯ���鷴Ӧ��2Cl2+2Ca(OH)2==CaCl3+CaClO2+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.��֪��4NH3+5O2 4NO+6H2O 4NO+3O2+2H2O��4HNO3
4NO+6H2O 4NO+3O2+2H2O��4HNO3
��������������������Ϊ0.20�������������Ϊ0.80�������������ռ�����
(1)a mol NO��ȫת��ΪHNO3��Ҫ����_______ mol
(2)ΪʹNH3ǡ����ȫ����Ϊһ����������������������а����������Ϊ________(����2λС��)��
(3)20.0 moL��NH3�ÿ����������������������Ϊ��NO 18.0 mol��O2 12.0 mol��N2 150.0 mol��һ���������ᣬ�Լ������ɷ֡�(������NO��O2����Ӧ)���㰱ת��ΪNO��HNO3��ת���ʡ�
(4)20.0 moL ��NH3��һ����������ַ�Ӧ����ת��ΪHNO3
������ͼ�л���HNO3�����ʵ���n(A)�Ϳ��������ʵ���n(B)��ϵ���������ߡ�
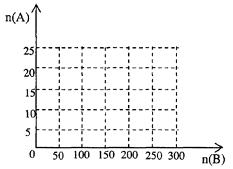
��д����125��n(B)��200ʱ��n(A)��n(B)�Ĺ�ϵʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������������������ԭ����һ����ȵ���(����)
A��������ȡ��ܶȲ��ȵ�N2��CO B���¶���ͬ�������ͬ��O2��N2
C�������ȡ��ܶ���ȵ�CO��C2H4 D��ѹǿ��ͬ�������ͬ��N2��O2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com