
����Ŀ�����������е�������ԭ�����α�����ԭ�ӻ����ȡ��ʱ�����γɵĻ�������������������Ҫ��N2H4(��)��HN3(�������⣬����ˮ��Ϊ������)��NH2OH(�ǰ�)��ˮ����(N2H4��H2O)���Ʊ���������(NaN3)��ԭ�ϣ���������������������ȫ��������������巢������ԭ�ϡ������ǹ�ҵˮ���·��Ʊ��������ƵĹ������̡�
���ϣ���ˮ�����ж��Ҳ��ȶ�������ǿ��ԭ�Ժ�ǿ���ԣ�
���й����ʵ������������±���
���� | �״� | ˮ���� | ��������� | �������� |
�۵�(��) | -97 | -40 | -17 | 275(410�棺�ֽ�) |
�е�(��) | 64.7 | 118.5 | -12 | �� |
�ش��������⣺
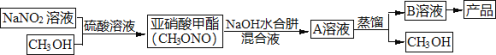
I.�ϳ�ˮ���¡�ʵ���Һϳ�ˮ����װ������ͼ��ʾ��NaClO������Һ������CO(NH2)2ˮ��Һ��400C���·�Ӧһ��ʱ�����Ѹ��������1100�������Ӧ�����Ƶ�ˮ���¡�
(1)ʵ����ͨ����Һ©��������ƿ�л����μ�NaClO������Һ�����ܷ���μӵ�ԭ����______________����ȡN2H4H2O�����ӷ���ʽΪ_______________________��
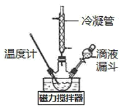
II.�Ʊ��������ơ�ʵ���ҿ�������ͼ����ʾ��װ�ü�ҩƷ�Ʊ��������ơ�
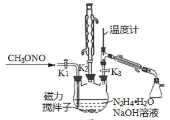
(2)�ٸ���ʵ�鷢���¶���20�����ҷ�Ӧ��ת������ߣ���˿��ȡ�Ĵ�ʩ��_______________������������A��Һʱ��װ��������K1��K2��K3�Ŀ��������_______________________��
��д���÷����Ʊ��������ƵĻ�ѧ����ʽ��________________________��
(3)��������B��Һ��õ������Ʋ�Ʒ��ʵ�鲽��Ϊ____________________����ѹ���ˣ��������Ҵ�ϴ��23�κ��
(4)���������У�����ĵ������Ƴ�ʹ�ô���������Һ�����������������£����߷�Ӧ�������������塣������6.5gNaN3�������������0.5molL��NaClO��Һ_____________mL��
III.�ǰ�(NH2OH)��һ�ֻ�ԭ��������ͨ�����й��̵õ�����ϩ��N2O4�����Ϻ��գ������ӳɷ�Ӧ���õ�������A��A�ṹ�Գƣ�������ͬԪ�ص�ԭ�ӻ�ѧ������ͬ��A��ijŨ�ȵ�������Һ�л��ɵõ�������B��ͬʱ�õ�CO��CO2��ɵĻ������(��������ܶ�Ϊ18)����������BΪ����̼Ԫ�ص������Σ��������Ԫ�ص����������ֱ�Ϊ19.51%��58.54%������A����CH3CH2NO2�������Ƶķ�Ӧ��Ҳ�ܵõ�B����û������ų���B��Һ���м��õ�NH2OH��
(5)NH2OH���������ԣ������ᷴӦ�����Σ����������ӵĵ���ʽΪ_______________��
(6)д��A��B�Ļ�ѧ����ʽ________________________��
���𰸡���ֹ������NaClO��Һ����ˮ���� ClO+CO(NH2)2+2OH=Cl+N2H4H2O+CO32 20����ˮԡ����ͨ������� �ر�K1��K2����K3 CH3ONO+N2H4H2O+NaOH�TCH3OH+NaN3+3H2O �����ᾧ 100 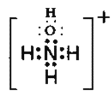 CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4
CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4
��������
ʵ���Һϳ�ˮ����װ������ͼ��ʾ��NaClO������Һ������CO(NH2)2ˮ��Һ��400�����·�Ӧһ��ʱ�����Ѹ��������1100�������Ӧ�����Ƶ�ˮ���£�������Ӧ�����ӷ���ʽΪ��ClO+CO(NH2)2+2OH=Cl+N2H4H2O+CO32��NaNO2��Һ��CH3OH�����������·�����Ӧ2CH3OH+2NaNO2+H2SO4(Ũ)=2CH3ONO+Na2SO4+2H2O�������������(CH3ONO)�����������(CH3ONO)����������ˮ���»��Һ��Ӧ���ɼ״��͵������ƣ���Ӧ����ʽΪ��CH3ONO+N2H4H2O+NaOH�TCH3OH+NaN3+3H2O����ϼ״��͵������Ƶ��۷е㲻ͬ����������ķ���������״��͵������ƣ���B��ҺΪ����������Һ�������нᾧ�����ˡ�ϴ�ӡ������ò�Ʒ�������ƣ��ݴ˷������I��II��
III����ϩ��N2O4�����Ϻ��գ������ӳɷ�Ӧ����A��A�Ľṹ�Գƣ�������ͬԪ�ص�ԭ�ӻ�ѧ������ͬ��������ӦΪ��CH2=CH2+ N2O4��CH2NO2CH2NO2���õ�������AΪCH2NO2-CH2NO2��A��ijŨ�ȵ�������Һ�л��ɵõ�������B��ͬʱ�õ�CO��CO2��ɵĻ������(��������ܶ�Ϊ18)�����Ϻ������ƽ����Է�������Ϊ18��2=36������ʮ����˷���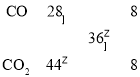 ����
����![]() ����������BΪ����̼Ԫ�ص������Σ��������Ԫ�ص����������ֱ�Ϊ19.51%��58.54%����B�к���Sԭ�Ӹ���Ϊx��Oԭ�Ӹ���Ϊy�� B��Ħ������ΪM����
����������BΪ����̼Ԫ�ص������Σ��������Ԫ�ص����������ֱ�Ϊ19.51%��58.54%����B�к���Sԭ�Ӹ���Ϊx��Oԭ�Ӹ���Ϊy�� B��Ħ������ΪM����![]() ��100%=19.51%��
��100%=19.51%��![]() ��100%=58.54%����ʽ����ɵã�
��100%=58.54%����ʽ����ɵã�![]() =
=![]() ����B�к���1��Sԭ��ʱ��Oԭ��Ϊ6�����������������ԭ�Ӹ�����Ϊ1��4��˵�������ӽṹ�к������� B����Է�������M=
����B�к���1��Sԭ��ʱ��Oԭ��Ϊ6�����������������ԭ�Ӹ�����Ϊ1��4��˵�������ӽṹ�к������� B����Է�������M=![]() =164����ΪNH2OH���������ԣ��ɽ��H+�õ�NH3OH+����BӦΪ(NH3OH)2SO4��(NH3OH)2SO4��Һ���з�Ӧ�õ�NH2OH��NH4+������A����CH3CH2NO2�������Ƶķ�Ӧ��Ҳ�ܵõ�B����û������ų�����Ӧ����ʽΪCH3CH2NO2+ H2SO4��(NH3OH)2SO4+C4H6O2���ݴ˷������
=164����ΪNH2OH���������ԣ��ɽ��H+�õ�NH3OH+����BӦΪ(NH3OH)2SO4��(NH3OH)2SO4��Һ���з�Ӧ�õ�NH2OH��NH4+������A����CH3CH2NO2�������Ƶķ�Ӧ��Ҳ�ܵõ�B����û������ų�����Ӧ����ʽΪCH3CH2NO2+ H2SO4��(NH3OH)2SO4+C4H6O2���ݴ˷������
I��(1)����������Ϣ��ˮ�����ж��Ҳ��ȶ�������ǿ��ԭ�Ժ�ǿ���ԣ�NaClO����ǿ�����ԣ�������ˮ���£�Ϊ��ֹ������NaClO����ˮ���£����ܷ���μӣ�NaClO��CO(NH2)2�ڼ��������·�Ӧ����ˮ���¡������Ӻ�̼������ӣ����ӷ���ʽClO+CO(NH2)2+2OH=Cl+N2H4H2O+CO32��
II��(2)�ٸ���ʵ�鷢���¶���20�����ҷ�Ӧ��ת������ߣ����Ǹ÷�Ӧ���ڷ��ȷ�Ӧ����˿��Բ�ȡ�Ĵ�ʩ�ǣ�20����ˮԡ����ͨ������ȣ�����������A��Һʱ����ҺA��������ĺ�������˳���ǣ��ر�K1��K2����K3��
�ڸ��ݷ�����֪���Ʊ��������ƵĻ�ѧ����ʽ��CH3ONO+N2H4H2O+NaOH�TCH3OH+NaN3+3H2O��
(3)��������B��Һ��õ������Ʋ�Ʒ��ʵ�鲽��Ϊ�����ᾧ����ѹ���ˣ��������Ҵ�ϴ��23�κ��
(4)����������NaN3Ӧ�ñ�����Ϊ����N2��ͬʱClO����ԭΪCl�����ÿ��NaN3�ڷ�Ӧ����Ҫʧȥ1�����ӣ�ÿ��ClO�ڷ�Ӧ�п��Եõ�2�����ӣ����ߵ����ʵ���֮��Ϊ2:1 �������ķ�ӦΪ��ClO+2N3-+2H+= Cl+3N2��+H2O��NaN3�����ʵ���n=![]() =0.1mol������ҪClO�����ʵ���Ϊ0.05mol�������Ҫ����������Һ���V=
=0.1mol������ҪClO�����ʵ���Ϊ0.05mol�������Ҫ����������Һ���V=![]() =0.1L=100mL��
=0.1L=100mL��
III��(5)�ǰ�(NH2OH)�ǰ�����������Կ��������е�һ����ԭ�ӱ��ǻ�ȡ�����γɵ����ʣ�NH2OH���������ԣ���Ȱ�����HCl����NH4Cl�ķ�Ӧ�����ǰ�(NH2OH)�����ᷴӦ�������ӻ�����HONH3Cl�������ӻ�����NH3OHCl��[NH3HO]+��Cl-���ɣ����������ӵĵ���ʽΪ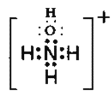 ��
��
(6)�����⣬A��ijŨ�ȵ�������Һ�л��ɵõ�������B�����ɵ�CO��CO2�����ʵ���֮��![]() ����Ӧ����ʽΪ��CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4��
����Ӧ����ʽΪ��CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4��


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(7��)ij����һ��˫������̬ϩ������һ��̬�����Ļ�����壬��ͬ��ͬѹ�����ܶ���H2��13��2�����ڱ�״���½�4��48L�������ͨ����������ˮ�������ˮ����3��36g����ԭ��������и�������������� (ע���������д�����̣����÷�)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ͳ��Ϊ��ɫ���������ǵĵ��ʡ��Ͻ��仯�����ڿ��к�������������Ҫ��;��
��ش��������⣺
��1����̬��ԭ�ӵļ۵��ӹ����ʾʽΪ__�������������������ڱ��е�___��Ԫ�ء�
��2����̬��ԭ�Ӻͻ�̬��ԭ���е�һ�����ܽϴ����___(��Ԫ�ط���)��ԭ��Ϊ___��
��3����ѧʽΪCrC13��6H2O�Ļ����������ֽṹ��һ�ֳ�������ɫ��һ�ֳʰ���ɫ��һ�ֳ�����ɫ����֪Cr3+����λ����Ϊ6�����������Ƴɵ������Ũ�ȵ���Һ���ֱ��������AgNO3��Һ������AgC1���������ʵ���֮��Ϊ3��2��1��
�ٳʰ���ɫ��������ڽ�Ļ�ѧʽΪ___��
��H2O���ӵ�VSEPRģ��Ϊ___��
��4��MnF2��MnCl2��Ϊ���ӻ����MnF2���۵����MnCl2�۵��ԭ��Ϊ___��
��5��һ��������ҽҩ��ұ������;�㷺�������������ṹ��ͼ��ʾ��
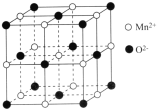
�ٸþ�������O2-�γɵ����������϶��ĿΪ__��
����NAΪ�����ӵ�������ֵ�������о������������O2-֮��ľ���Ϊapm����MnO������ܶ���=____g��cm-3��(�ú�a��NA�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪0.1 mol��L��1�Ĵ�����Һ�д��ڵ���ƽ�⣺CH3COOH![]() CH3COO����H����Ҫʹ��Һ��
CH3COO����H����Ҫʹ��Һ��![]() ֵ�����Բ�ȡ�Ĵ�ʩ��
ֵ�����Բ�ȡ�Ĵ�ʩ��
A.�������ռ�B.�����¶�C.������������D.��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ���ʵ��̽����ҵ����ϩ��ԭ������ϩ����Ҫ��ѧ���ʣ�ʵ��װ����ͼ��ʾ(��֪�������Ӧ)����ش��������⣺

��1����ҵ����ϩ��ʵ��ԭ��������(Һ̬)�ڴ����ͼ��������·�����Ӧ���ɲ������������磬ʯ�ͷ������֮һ��ʮ������������Ӧ��C16H34![]() C8H18���ף���
C8H18���ף���![]() 4�ң���ķ���ʽΪ________���ҵĽṹ��ʽΪ____________________________________��
4�ң���ķ���ʽΪ________���ҵĽṹ��ʽΪ____________________________________��
��2��Bװ���е�ʵ�����������________��д����Ӧ�Ļ�ѧ����ʽ��__________���䷴Ӧ������________��
��3��Cװ���пɹ۲쵽��������____________����Ӧ������________��
��4����������֪����ϩ�����Ը��������Һ��Ӧ����������̼�����ݱ�ʵ����װ��_____(����ĸ)�е�ʵ��������жϸ������Ƿ���ʵ��Ϊ��̽��������ϩ��Ӧ�Ǽӳɷ�Ӧ������ȡ����Ӧ�����Բⶨװ��B����Һ�ڷ�Ӧǰ�������ԣ����������ɣ� __________________��
��5��ͨ������ʵ��̽��������������ϩ�ķ�����________(ѡ����ĸ����ͬ)����ȥ��������ϩ�ķ�����________��
A������ͨ��ˮ�� B������ͨ��ʢ��ˮ��ϴ��ƿ
C������ͨ��ʢ���Ը��������Һ��ϴ��ƿ D������ͨ������������Һ
��6���ֱ�ȼ����ϩ�ͼ��飬���������������ϩ�����������̣�ԭ����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������Ӧ�÷dz��㷺��2NH3(g)+CO2(g)CO(NH2)2(s)+H2O(l)�ǹ�ҵ�Ϻϳɵ������ص���Ҫ�����������������£���λʱ���ڻ�����صIJ�����ѹǿ��n(NH3)��n(CO2)�Ĺ�ϵ��ͼ����ʾ������̼��n(NH3)��n(CO2)=4ʱ��CO2��ת������ʱ��ı仯��ϵ��ͼ����ʾ������˵������ȷ����
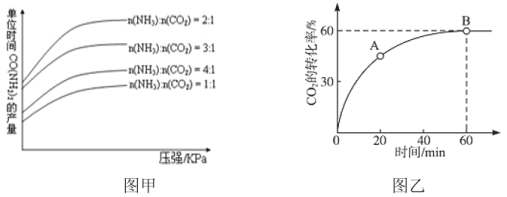
A.��NH3��CO2��ʾ���ʣ�v(NH3)��v(CO2)=2:1
B.����ʼͶ�ϰ���n(NH3)��n(CO2)Ϊ2��1Ͷ�ϣ�ƽ�����ѹ����������������ٴ�ƽ��ʱc(NH3)��ѹ��ǰС
C.A����淴Ӧ����С��B�������Ӧ����
D.��ͼ�ҿ�֪NH3��ƽ��ת����Ϊ30��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ����˹�����ǣ���ͼ�ף��ܹ���ú������еļ���ﵽһ��Ũ��ʱ��ͨ����������ʾ����������˹�����ǹ���ԭ������ȼ�ϵ�صĹ���ԭ������װ������ͼ����ʾ�����еĹ���������Y2O3��Na2O��O2�����������������ƶ��������й�������ȷ�ĵ���( )
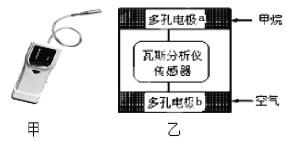
A. ��˹�����ǹ���ʱ������ڵ�·�е����ɵ缫b����缫a
B. �缫a�ķ�ӦʽΪ��CH4+5O2���D8e����CO32�� +2H2O
C. �缫b������, O2-�ɵ缫a����缫b
D. ��������������1 mol O2��ͨ��ʱ������ת��4 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�ڷ�ӦA(g)+3B(g)��2C(g)�У���������A��ʾ�ĸ÷�Ӧ�Ļ�ѧ��Ӧ����Ϊ0.2 mol��L1��min1����������B��ʾ�˷�Ӧ�Ļ�ѧ��Ӧ����Ϊ________mol��L1��min1��
(2)��2 L���ܱ������У�����2 mol N2��3 mol H2����һ�������·�����Ӧ��3 s����N2Ϊ1.9 mol������H2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ____________________________��
(3)��10 mol A��5 mol B�����ݻ�Ϊ10 L���ܱ������У�ij�¶��·�����Ӧ��3A(g)+B(g) ![]() 2C(g)�������2 s�ڣ�����A��ƽ������Ϊ0.06 mol��L1��s1������2 sʱ����������______ mol A����ʱC�����ʵ���Ũ��Ϊ________��
2C(g)�������2 s�ڣ�����A��ƽ������Ϊ0.06 mol��L1��s1������2 sʱ����������______ mol A����ʱC�����ʵ���Ũ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2.0molPCl3��1.0molCl2�������������ܱ������У���һ�������·���������Ӧ��PCl3(g)+Cl2(g)PCl5(g)��ƽ��ʱ��PCl5Ϊ0.4mol�������ʱ����1.0molPCl3��0.50molCl2������ͬ�¶����ٴ�ƽ��ʱPCl5�����ʵ����ǣ� ��
A. 0.4mol
B. 0.2mol
C. ��0.2mol
D. ����0.2mol����0.4mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com