
��10�֣�
��1����һ�鴿����пƬ����ʢ��ϡ������ձ���ɹ۲쵽пƬ���ܽ⣬���������������ƽ�еز���һ��ͭƬ����ͼ����ʾ�����ɹ۲쵽ͭƬ�� ����С���û�С������ݲ��������õ��߰�пƬ��ͭƬ������������ͼ����ʾ�����ɹ۲쵽ͭƬ�� ����С���û�С������ݲ�����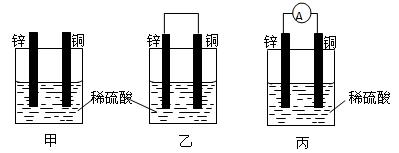
��2���õ����������������������˺�������Һ�е�пƬ��ͭƬ��������ͼ����ʾ�����۲쵽������������ָ�뷢����ƫת��˵���˵������е���ͨ����ͼ�ҡ�ͼ����һ���� ��ת��Ϊ �ܵ�װ�ã� ���ǰ�������ԭ��ء�
���ǰ�������ԭ��ء�
��3�������������п��Թ��ɳ�����ԭ��ص�һЩ������ ���йصĵ缫��Ӧʽ��пƬ ��ͭƬ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��10�֣�
��1����һ�鴿����пƬ����ʢ��ϡ������ձ���ɹ۲쵽пƬ���ܽ⣬���������������ƽ�еز���һ��ͭƬ����ͼ����ʾ�����ɹ۲쵽ͭƬ�� ����С���û�С������ݲ��������õ��߰�пƬ��ͭƬ������������ͼ����ʾ�����ɹ۲쵽ͭƬ�� ����С���û�С������ݲ�����
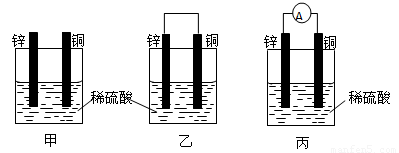
��2���õ����������������������˺�������Һ�е�пƬ��ͭƬ��������ͼ����ʾ�����۲쵽������������ָ�뷢����ƫת��˵���˵������е���ͨ����ͼ�ҡ�ͼ����һ���� ��ת��Ϊ �ܵ�װ�ã����ǰ�������ԭ��ء�
��3�������������п��Թ��ɳ�����ԭ��ص�һЩ������ ���йصĵ缫��Ӧʽ��пƬ ��ͭƬ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com