
����Ŀ��������(Mo)����(W)���ǵڢ�B��Ԫ�أ���ԭ�����������������ǵĵ��ʺͻ�����������������й㷺Ӧ�á��ش��������⣺
��1����Ԫ�ص�����ϼ�Ϊ_________����̬��ԭ�ӵĺ�������Ų������ڻ�̬��ԭ�ӣ����̬��ԭ�Ӻ�����_________��δ�ɶԵ��ӣ���̬��ԭ�ӵĺ�������Ų��ǡ����ع������⣬���̬��ԭ�Ӽ۵��Ӳ�ĵ����Ų�ͼΪ____________________��
��2��������л��ϳɵĴ��������磬����ȩ����ԭ�ɻ������״���
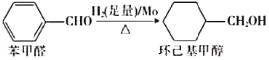
�ٱ���ȩ�����������_________________��ԭ�ӹ�ƽ�档
�ڻ������״������в�ȡsp3�ӻ���ԭ����_____________(дԪ�ط���)��
��3��������(Cr3��)���γɶ�����������[Cr(OH)3(H2O)(H2NCH2CH2NH2)]��
����֪�������������ӵ���λ��ָ��λԭ������������������У�Cr3������λ��Ϊ______��
������������У��ǽ���Ԫ�صĵ縺����С�����˳��Ϊ____________��
��4������һ��������ľ����ṹ��ͼ��ʾ��
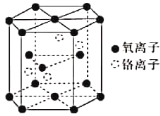
�ٸ�������Ļ�ѧʽΪ____________________��
����֪�������ױ߱߳�Ϊacm����Ϊbcm�������ӵ�������ֵΪNA���þ�����ܶ�Ϊ___________gcm��3(�г�����ʽ)��
���𰸡�+6 6 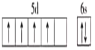 14 C��O 6 H��C��N��O Cr2O3 4
14 C��O 6 H��C��N��O Cr2O3 4![]() ��152/��9NA��a2b��
��152/��9NA��a2b��
��������
��1����Ԫ�صļ۵����Ų�ʽΪ3d54s1��������ϼ�Ϊ+6�ۣ���̬��ԭ�ӵĺ�������Ų������ڻ�̬��ԭ�Ӽ۵����Ų�ʽΪ4d55s1����6��δ�ɶԵ��ӣ���̬��ԭ�ӵĺ�������Ų��ǡ����ع������⣬�۵��Ӳ�ĵ����Ų�ͼΪ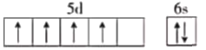 ��
��
��2������������ȩ��ȷ����2��ƽ���غ�ʱ������14ԭ�ӹ�ƽ�棻
�ڻ������״������У�����Cԭ��Ϊ4�����ۼ����µ��Ӷԣ�Oԭ����2������2���µ��Ӷԣ�Ϊsp3�ӻ���
��3����������е�����ΪOH��H2O��H2NCH2CH2NH2������O��Nԭ���ṩ�µ��Ӷ��γ���λ��������λ��Ϊ6��
������������У��ǽ���Ԫ����H��C��N��O���縺�Ե���С�����˳��ΪH��C��N��O��
��4���ٸ��ݸ�������ľ�����֪��Oԭ���ھ����Ķ��㡢�������ĺ����ڣ�����=12��1/6+2��1/2+3=6�������������ڣ�����Ϊ4������֮��Ϊ6��4=3��2����ѧʽΪCr2O3��
����=m/V=��52��2+16��3����2/��a��![]() a��b��NA��=4
a��b��NA��=4![]() ��152/��9NA��a2b����
��152/��9NA��a2b����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��100��ʱ����ij�����ܱ������м���1.6 mol ��L��1��Q��ᷢ�����·�Ӧ��2Q(g) ![]() M(g) ������M�����ʵ���Ũ����ʱ��ı仯��ͼ��ʾ��
M(g) ������M�����ʵ���Ũ����ʱ��ı仯��ͼ��ʾ��
����˵���������
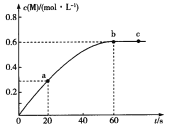
A.�ӷ�Ӧ��ʼ���մﵽƽ��ʱ����ڣ�v(Q)��0.02 mol��L��1��s��1
B.a��b��ʱ������Q�����ʣ�v(a)��v(b)
C.��QŨ�ȱ仯ֵ��ʾ��ab��bc����ʱ���ڵķ�Ӧ���ʣ�v(ab)��v(bc)��0
D.����������ͬ����ʼʱ��0. 2 mol��L��1������Q��ϣ���Ӧ�ﵽƽ������ʱ������60 s
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ʵ��ʱ������þʧ�����������������̼��������ѻ�����ȴ��ʵ����ʦ��ʱ��ֹ��ԭ����CO2����֧��þȼ�շ������·�Ӧ��2Mg+CO2![]() 2MgO+C�����й��ڸ÷�Ӧ���ж���ȷ����
2MgO+C�����й��ڸ÷�Ӧ���ж���ȷ����
A. MgԪ�ػ��ϼ���0�����ߵ�+2�ۣ�����MgO�ǻ�ԭ����
B. �ɴ˷�Ӧ�����ж�������CO2>MgO����ԭ��Mg>C
C. CO2�������������������ԣ�����������Ӧ
D. Mgԭ��ʧȥ�ĵ�����Ŀ����Oԭ�ӵõ��ĵ�����Ŀ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���A�ǾۺϷ�Ӧ�����������ϵĵ��壬�����Ϊ�ϳɵ����I����������J��ԭ�ϣ���غϳ�·�����£�

��֪��������ͼ����A������ʺɱ�Ϊ118���䱽���ϵ�һ�ȴ��ﹲ���֣��˴Ź���������
ʾ�������Ϊ3:2:2:2:1��
����������Ϣ�ش��������⣺
��1��A�Ĺ���������Ϊ__________________��B��C�ķ�Ӧ����Ϊ_____________��E��F�ķ�Ӧ����Ϊ_____________��
��2��I�Ľṹ��ʽΪ____________________����K�����к���������Ԫ��״�ṹ���������ʽΪ________________��
��3��D������������ͭ����Һ��Ӧ�����ӷ���ʽΪ_______________________________��
��4��H��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������1 mol W���뷴Ӧ�������3 mol Br2����д�����з���������W�Ľṹ��ʽ___________________________________��
��5��J��һ�ָ߷��ӻ��������C����J�Ļ�ѧ����ʽΪ
______________________________________________________________________��
��6��![]() ��֪��
��֪��![]() ��RΪ������
��R������
����Ա�����ϩΪ��ʼԭ���Ʊ�H�ĺϳ�·�ߣ����Լ���ѡ����
[�ϳ�·��ʾ����]![]()
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ҫ��ش��������⣺
I.��ϵ���������������й㷺Ӧ�á�
��1��NaClO2������ˮ��������������FeSO47H2O����������������ơ�
��Fe2���ĵ����Ų�ʽΪ[Ar]________________________________��
����SO42����Ϊ�ȵ�����ķ�����____________________(дһ��)��
��ClO2������ԭ�ӵŵ��Ӷ���Ϊ___________��
�ܳ���K3[Fe(CN)6]����ˮ�е�F2����K3[Fe(CN)6]��������________________��
��2��ClO2����һ������ˮ���������е㣺ClO2________________(���������������)Cl2O��������________________________________________________��
��.Fe��CuΪ���ɽ���Ԫ�أ������ڹ�ҵ�����ж�����Ҫ��Ӧ�á�
��2�����Ҵ�����ͨ�����ȵ�����ͭ��ĩ���ᷢ����Ӧ��![]()
����ͬѧ��д��̬̼ԭ�ӵĺ�������Ų�ͼΪ![]() ����������д����ȷ��Υ����________��
����������д����ȷ��Υ����________��
����ȩ���Ҵ�����Է����������2�������Ҵ��ķе�Զ������ȩ������Ҫԭ����_______��
��2��Fe��Fe2�����ܱ�����������HNO3�е�ԭ�ӹ�����ӻ�����Ϊ_______________________��
��3��NO�ܱ�FeSO4��Һ�������������![]() ����������������ӵ���λ��Ϊ__________________________��
����������������ӵ���λ��Ϊ__________________________��
��4���о����֣������ӵ���ɫ��δ�ɶԵ������йء����磬Cu2����Fe2����Fe3���ȡ�Cu������ɫ����ԭ����_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ͻ�ԭ�������������й㷺ʹ�á�
��1��H3PO2���ɽ���Һ�е� Ag����ԭΪ Ag���Ӷ������ڻ�ѧ������
��H3PO2��PԪ�صĻ��ϼ�Ϊ___��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�����ʵ���֮��Ϊ4��1������������Ϊ___��
��2��������һ��ǿ�ᣬ�����Ũ�ȳ���40%�ͻ�Ѹ�ٷֽ⣬��Ӧ�Ļ�ѧ����ʽΪ8HClO3��3O2����2Cl2����4HClO4��2H2O�����������������С�⣺
�ٸ÷�Ӧ�Ļ�ԭ������___(�ѧʽ)��
�����û�������ƽ����Է�������Ϊ___��
��3����֪�ⶨ�̵�һ�ַ����ǣ�������ת��Ϊ����������ӣ���Ӧ��ϵ����H����Mn2����H2O��IO3����MnO4����IO4����
���йط�Ӧ�����ӷ���ʽΪ___��
����������ת��Ϊ����������ӵķ�Ӧ�У�����ѷ�Ӧ�����Һϡ�͵�1L�������Һ��c(H��)��0.03mol/L�����ڷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ___mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ƿ�������ʱ���õ����������Խ��еĻ�����������ֱ��ǣ���
![]()
A.�����ˡ���ȡ������B.������������ȡ������
C.��ȡ�����ˡ���������D.���ˡ���������ȡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺

������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2��Na2S2O5��
��1�����Լ�ǰҪ���еIJ�����_______________��
��2��Ũ����_______ (��ܡ����ܡ�)��ϡ������棬ԭ����______________��
��3����װ�â��з������Ʒ�ɲ�ȡ�ķ��뷽����_____��
��4��Ϊ������ʵ��װ�ã�������װ����ѡ��һ���������װ�÷���װ�â���ѡ�õ�װ��(�г���������ȥ)Ϊ___________(�����)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶���,�ڹ̶�������ܱ������з������з�Ӧ:2HI![]() H2+I2����c(HI)��0.1 mol��L-1����0.07 mol��L-1ʱ,��Ҫ15 s,��ôc(HI)��0.07 mol��L-1����0.05 mol��L-1ʱ,���跴Ӧ��ʱ��Ϊ (����)
H2+I2����c(HI)��0.1 mol��L-1����0.07 mol��L-1ʱ,��Ҫ15 s,��ôc(HI)��0.07 mol��L-1����0.05 mol��L-1ʱ,���跴Ӧ��ʱ��Ϊ (����)
A. 5 sB. 10 sC. ����10 sD. ��10 s
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com