
���� ��1����SO2ʹCr2O72-ȫ��ת��ΪCr3+������3SO2����Cr2O72-�����ݹ�ϵʽ���������������
��Ksp[Cr��OH��3]=c��Cr3+��•c3��OH-��=6.3��10-31����ʹ��Һ�в���Cr3+�����ʵ���Ũ��Ϊ6.3��10-7 mol•L-1������c��OH-��=$\root{3}{\frac{6.3��1{0}^{-31}}{6.3��1{0}^{-7}}}$=10-8mol/L��Ӧ������Һ��pOH=8����pH=6��
��2����������в������������NH4��2S2O8����Cr2O72-��������ⶨ�ĸ�Ԫ����Ũ�Ȼ�ƫ�ߣ�
���ɢ�2Cr3++3S2O82-+7H2O=Cr2O72-+6SO42-+14H+
��Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O
��I2+2S2O32-=2I-+S4O62-�ã�Cr2O72-��3I2��6S2O32-���ɴ˷������
��� �⣺��1����SO2ʹCr2O72-ȫ��ת��ΪCr3+��
����3SO2����Cr2O72-��
3��22.4L 1mol
V 1000L��0.002mol•L-1
����V=$\frac{1000L��0.002mol•{L}^{-1}��3��22.4L}{1mol}$=134.4L���ʴ�Ϊ��134.4��
��Ksp[Cr��OH��3]=c��Cr3+��•c3��OH-��=6.3��10-31����ʹ��Һ�в���Cr3+�����ʵ���Ũ��Ϊ6.3��10-7 mol•L-1������c��OH-��=$\root{3}{\frac{6.3��1{0}^{-31}}{6.3��1{0}^{-7}}}$=10-8mol/L��Ӧ������Һ��pOH=8����pH=6���ʴ�Ϊ��6��
��2����������в������������NH4��2S2O8����Cr2O72-��������ⶨ�ĸ�Ԫ����Ũ�Ȼ�ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
���ɢ�2Cr3++3S2O82-+7H2O=Cr2O72-+6SO42-+14H+
��Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O
��I2+2S2O32-=2I-+S4O62-�ã�Cr2O72-��3I2��6S2O32-�����ݷ���ʽȷ����ϵCr2O72-��3I2��6S2O32-
n��Cr2O72-��=$\frac{n��{S}_{2}{O}_{3}^{2-}��}{6}$=$\frac{0.03��20.00��1{0}^{-3}}{6}$mol������c��Cr2O72-��=$\frac{\frac{0.03��20��1{0}^{-3}}{6}}{20��1{0}^{-3}}$=0.005mol/L
���Է�ˮ�и�Ԫ����Ũ��=0.005��2��52��1000=520 mg•L-1����Cr2O72-��3I2��6S2O32-�����ݷ���ʽȷ����ϵCr2O72-��3I2��6S2O32-
n��Cr2O72-��=$\frac{n��{S}_{2}{O}_{3}^{2-}��}{6}$=$\frac{0.03��20.00��1{0}^{-3}}{6}$mol������c��Cr2O72-��=$\frac{\frac{0.03��20��1{0}^{-3}}{6}}{20��1{0}^{-3}}$=0.005mol/L
���Է�ˮ�и�Ԫ����Ũ��=0.005��2��52��1000=520 mg•L-1��
���� ���⿼��������ԭ��Ӧ�ĵζ�ԭ����ѧ��Ҫѧ����ݷ�Ӧ����ʽ��ʽ������ܶȻ������֪ʶ����һ�����Ѷȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��ɫ��Һ�п��ܴ�������H+��Cl-��MnO4- | |
| B�� | pH=2����Һ�п��ܴ�������Na+��NH4+��SiO32- | |
| C�� | �������������Һ��ͨ��������Cl2+2OH-�TClO-+Cl-+H2O | |
| D�� | ϡ���������������Һ��Ӧ��H++SO42-+Ba2++OH-=BaSO4��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������ʽ�� | B�� | F-�Ľṹʾ��ͼ�� | ||
| C�� | ���ǻ�������Ľṹ��ʽ�� | D�� | ������Ϊ28�ĸ�ԭ�ӣ�${\;}_{20}^{28}$Ca |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
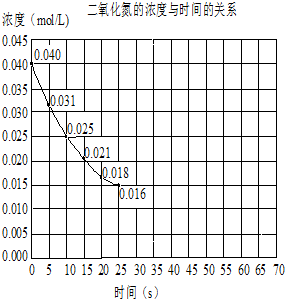
 ��������о���Ա������һ�ֿɿ��ٳ�ŵ�������ӵ�أ��õ�ص����Ϊ����Һ��{AlCl3/[EMIM]Cl}���ŵ�ʱ�й�����ת����ͼ��ʾ������˵����ȷ���ǣ�������
��������о���Ա������һ�ֿɿ��ٳ�ŵ�������ӵ�أ��õ�ص����Ϊ����Һ��{AlCl3/[EMIM]Cl}���ŵ�ʱ�й�����ת����ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �ŵ�ʱ������������2AlCl4--e-=Al2Cl3-+Cl- | |
| B�� | ���ʱ����ĭʯī�������Դ�ĸ������� | |
| C�� | �ŵ�ʱ����·��ÿ����3mol���ӣ���������27g | |
| D�� | ���ʱ������������4Al2Cl3-+3e-=Al+7AlCl4- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 28gMgCO3��NaHCO3��Ϲ����е�CO32-������ΪNA | |
| B�� | lmolI2��4molH2��Ӧ���ɵ�HI������Ϊ2NA | |
| C�� | 1molAl���ں�1molNaOH��Һ������ת����Ϊ3NA | |
| D�� | ��״���£�2.24LH2O���еĹ��ۼ���Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ�������ʹ�����Ȼ�̼ʱ���������� | |
| B�� | ʵ���������ȡǿ�ᡢǿ����ҺӦ����������� | |
| C�� | ʵ�鳡���Ͻ�Я��ʳ���ֹ������ƿװ��ѧҩƷ����ֹ��ʳ | |
| D�� | ��ȡ��ĩ״���ж�ҩƷʱ��Ҫ�����ַ�ֹ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
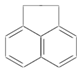 ij�л���ṹʽ��ͼ��ʾ����һ�ȴ��ﹲ�У������������칹����������
ij�л���ṹʽ��ͼ��ʾ����һ�ȴ��ﹲ�У������������칹����������| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 7�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com