
����Ŀ��ij����С�齫������Ĺ�ҵ������������Դ�����ã�����õĵ���������ȡ��84������Һ����֪��2H2S(g)+O2(g)= S2(s)+2H2O��1����H=-632kJ��mol-1����ͼΪ��С����Ƶ�ԭ��ͼ������˵����ȷ����

A. �缫a Ϊȼ�ϵ������
B. �缫b�Ϸ����ĵ缫��ӦΪ��O2+4e-+2H2O= 4OH-
C. ��·��ÿ����4mol ���ӣ�����ڲ��ͷ�����С��632 kJ
D. a��ÿ����32g��������e ���ռ�������22.4 L
���𰸡�C
��������A���缫a��H2S����ʧ���ӵ�������Ӧ����S2���缫aΪȼ�ϵ�صĸ�����A�����B���缫b��ͨ��O2���缫bΪ�������缫��ӦʽΪO2+4e-+4H+=2H2O��B�����C����·��ÿ����4mol������2molH2S��1molO2���뷴Ӧ���ͷ�����632kJ���˹��̽���ѧ��ת��Ϊ���ܺ����ܣ�����ڲ��ͷ�����С��632kJ��C����ȷ��D��a���缫��ӦʽΪ2H2S-4e-=S2+4H+��ʯī�缫c��缫a������ʯī�缫cΪ�������缫c�ĵ缫��ӦʽΪ2H2O+2e-=H2��+2OH-��������e�ռ���������ΪH2�����ݵ����غ㣬4n��S2��=2n��H2����n��H2��=2n��S2��=2![]() =1mol������H2�����¶Ⱥ�ѹǿδ֪�������㵼��e�ռ���H2�������D�����ѡC��
=1mol������H2�����¶Ⱥ�ѹǿδ֪�������㵼��e�ռ���H2�������D�����ѡC��


 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڽ����˵������ȷ���ǣ� ��
A.������۲�����
B.������ͨ����Ĥ
C.����������ͣ���������˶�
D.���岻�ȶ������ú����ײ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A�ķ���ʽΪC2H2,B�Ǻϳ����ϵĵ��壬C����Է�������Ϊ78,M��һ�־��й���ЧʳƷ�������õ��л���,��AΪԭ�Ϻϳ�B��M��·����ͼ��ʾ��
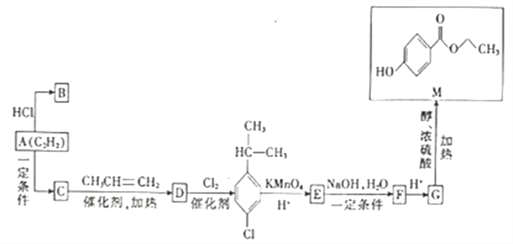
��1��C��D�ķ�Ӧ����Ϊ___________��B�Ľṹ��ʽΪ___________��
��2��д��E��F�Ļ�ѧ����ʽ��_______________________________��
��3��G�еĺ��������ŵ�������_______________,д����G��һ�������·�Ӧ���ɸ߷��ӻ�����Ļ�ѧ��Ӧ����ʽ��_______________________________________��
��4��M��ͬ���칹���ж���,д��������������������ͬ���칹��Ľṹ��ʽ��___________��___________��___________��___________��
���ܷ���������Ӧ
�������ͻ��ұ�����һ��ȡ����������
����FeCl3��Һ������ɫ
��1mol���л������������Ʒ�Ӧ����1mol����(һ��̼ԭ����ͬʱ��������-OH�Ľṹ���ȶ�)
��5���Լ�����![]() ����Ҫ���л������м��壬д������Ȳ����ȲΪԭ��(���Լ���ѡ)�Ʊ��Լ����ӵĺϳ�·�ߣ�_________________________________________________��
����Ҫ���л������м��壬д������Ȳ����ȲΪԭ��(���Լ���ѡ)�Ʊ��Լ����ӵĺϳ�·�ߣ�_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л����У��������������������(����)
A��±���� B������
C���ڶ��ױ� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʯ��Һ��������Ҫ�ɷ��Ƕ��飬��ʹ�ù����У�����һЩ������Һ̬�����ڸ�ƿ�У���Щ���ʿ����ǣ� ��
A. ����ͱ��� B. ����ͼ�ȩ C. ����Ͷ��� D. ����ͼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��
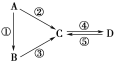
(1)��A�ǵ���ɫ���壬C��D�������C������������Ҫ���ʣ���CҲ����㷺����;��д�����е�������;��______________________________��
(2)��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�������Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
(3)��D���ʾ������ԣ��ڡ��۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧʱͨ�������һ����������ЧӦ����Ҫ���塣����A���ڿ������ȶ����ڵ�ԭ����_______________________________________________________��
(4)��A��̫���ܵ���õĹ�����ϡ�C��DΪ���Σ�C��һ�ֿ��コ�������������D��Һ�Լ��ԣ������ڹ�ҵ����������д���ڷ�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��D�Ļ�ѧʽ��________��
(5)��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڡ��ݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬д���÷�Ӧ�����ӷ���ʽ��____________________________________________________________��
(6)��BΪ����ɫ���廯���D�������࣬��ˮ��Һ�Լ��ԣ��ڡ��۷�Ӧ��Ҫ�õ�һ����ɫҺ�壬д����Ӧ�ݵĻ�ѧ����ʽ��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������У�A��KI��s����B��C2H5OH��l����C��Cl2��g����D��CH3COOH��l����E��BaSO4��s����F��NaHSO4��s����G��ʯī��s����H��������s����I��NaOH��l��
��1�����ڷǵ���ʵ���____________������ţ���ͬ��������ǿ����ʵ���____________������������ʵ���____________��
��2����ֱ�ӵ������________________��������ˮ����ˮ��Һ�ܵ������________________��
���� ����������ʵ�������ص����ӷ���ʽ����뷽��ʽ
��1��Na2S��Һʹ��̪�Ժ�ɫ_________________________________��
��2����������[����KAl��SO4��2��12H2O]��������ˮ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ����Ҫ�Ļ���ԭ�ϡ����ұ�(C6H5��CH2CH3)Ϊԭ�ϣ����ô�����ķ�����ȡ����ϩ(C6H5��CH=CH2)����Ӧ����ʽΪ��C6H5��CH2CH3(g)![]() C6H5��CH=CH2(g)+H2(g) ��H=+117.6 kJ��mo1-1
C6H5��CH=CH2(g)+H2(g) ��H=+117.6 kJ��mo1-1
�ش�����������
��1����֪��H2(g)+1/2O2(g)=H2O(l) ��H=-285.8 kJ��mo1-1
C6H5��CH2CH3(g)+21/2O2(g)=8CO2(g)+5H2O(l) ��H=-4607.1 kJ��mo1-1
��C6H5��CH=CH2(g)+10O2(g)= 8CO2(g)+4H2O(l) ��H=_____________��
��2����ҵ�ϣ��ں�ѹ�豸�н���������Ӧ��ȡ����ϩ�������ұ�������ͨ�����ˮ���������û�ѧƽ�����۽���ͨ�����ˮ������ԭ��_________________________________________��
��3����֪T���£���amol�ұ�����ͨ�뵽���ΪVL���ܱ������н���������Ӧ����Ӧʱ���������ڵ���ѹǿ�������±���
ʱ��t/min | 0 | 10 | 20 | 30 | 40 |
��ѹǿp/1000kPa | 1.0 | 1.3 | 1.45 | 1.5 | 1.5 |
���ɱ������ݼ���0~10 min��v(C6H5��CH2CH3)=________________�����ú�a��V ��ʽ�ӱ�ʾ��
���÷�Ӧƽ��ʱ�ұ���ת����Ϊ_________________________��
��4������ϩ���廯�ⷢ���ļӳɷ�Ӧ���������֣��䷴Ӧ����ʽ������
i.C6H5��CH=CH2(g)+HBr(g)![]() C6H5��CH2CH2Br (g)
C6H5��CH2CH2Br (g)
ii.C6H5��CH=CH2(g)+HBr(g)![]() C6H5��CHBrCH3(g)
C6H5��CHBrCH3(g)
600��ʱ����3L �����ܱ������г���1.2 mol C6H5��CH=CH2(g)��1.2 mol HBr(g)������Ӧ���ﵽƽ��ʱC6H5��CH2CH2Br (g)��C6H5��CHBrCH3(g)�����ʵ���(n)��ʱ��(t)�仯��������ͼ��ʾ��
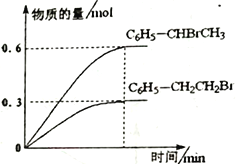
��600��ʱ����Ӧii �Ļ�ѧƽ�ⳣ��K ii=__________________��
����Ӧƽ��������������������䣬����������ٳ���1mol C6H5��CH2CH2Br (g)����Ӧii ��_________(��������������)�ƶ���
���ں��º��ݵ��ܱ������У�����ϩ���廯�ⷢ��i��ii�����ӳɷ�Ӧ���жϷ�Ӧ�Ѵﵽƽ��״̬����______��
B.C6H5��CH2CH2Br (g)������������C6H5��CHBrCH3 (g)�ֽ��������
C.��Ӧ����ѹǿ������ʱ��仯���仯
D.��������ƽ����Է����������ֲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�����ڱ��У�����22�ַǽ���Ԫ���⣬����Ķ��ǽ����������Ԫ�����ڱ��ش��������⣺
I��(1)��̬��ԭ�Ӻ����_______���˶�״̬����ͬ�ĵ��ӣ���ԭ�Ӻ�������Ų��е�����������͵Ĺ����_______�Σ���n��ʾ�ܲ㣬��Ԫ�����������Χ�����Ų�ʽΪ______________��
(2)��Ԫ�����ڱ��У�ijЩ����Ԫ�����·�������Ԫ�ص�������Щ���ƣ�����Ϊ���Խ��߹������±���

���ݡ��Խ��߹���д��Be(OH)2��NaOH��Ӧ�����ӷ���ʽ______________������(H3BO3)��һ�־���Ƭ��ṹ�İ�ɫ���壬���ڵ�H3BO3���Ӽ�ͨ�������������ͼ������1mol H3BO3�ľ�������__________mol�����H3BO3��Bԭ�ӵ��ӻ�����Ϊ_____________��

(3)�Եڶ�����Ϊ������Be��N�⣬����Ԫ�صĵ�һ�����ܴ������������ԭ����____________________________________________________��
II�������������仯�����ڹ�ũҵ���й�����Ӧ��ǰ����
(4)����һ��������[Fe(CN)6]4-��Fe2+����λ��Ϊ6�����������в�����______������ţ���
A�����ۼ� B���Ǽ��Լ� C����λ�� D���ļ� E���м�
(5)AlCl3���۵��NaCl�۵�͵�ԭ����____________________________________��
(6)һ��Al-Fe�Ͻ�����徧����ͼ��ʾ����������ܶ�Ϊ�� gcm-3����˺Ͻ������������Feԭ��֮��ľ���Ϊ__________cm���ú����Ĵ���ʽ��ʾ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com