
ʵ�����У�������ͼ��ʾװ�ü�����ҩƷ��ͼ�в��ּг���������ȥ��̽����ҵ������Ӵ����еķ�Ӧ�����ⶨ�������¶��������ת���ʡ���֪SO3�۵�Ϊ16��8�棬�����������װ��ʱ�ֱ���ȫ���գ��Һ���װ���ڿ����е�CO2��
��1����֪0��5molSO2��O2��������̬SO3���ų�49��15 kJ��������Ӧ���Ȼ�ѧ����ʽΪ ��
��2������ʵ��Ŀ�ģ����������ͼ��ѡ����ʵ�װ�ã������������ո��У�װ�â� ��װ�â� ��װ�â� ��
��3����ʼ����ʵ��ʱ������Ӧ���еIJ����� ��
��4������Ӳ�ʲ�����ʱ�������������¶ȣ�SO2��ת���ʻ� ������������䡱��С������
��5��ֹͣͨ��SO2��Ϩ��ƾ��ƺ�Ϊʹ������װ���е�SO2��SO3��������գ����������� ��
��6��ʵ���������װ�â����ӵ�����Ϊb g ,װ�â����ӵ�����Ϊa g����������¶��������ת������ ���ú���ĸ�Ĵ�����ʾ����
��1��2SO2��g��+O2��g��  2SO3��g������H=��196��6 kJ/mol ��2�֣�
2SO3��g������H=��196��6 kJ/mol ��2�֣�
�� SO2��g��+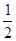 O2��g��
O2��g��  SO3��g������H=��98��3 kJ/mol
SO3��g������H=��98��3 kJ/mol
��2�� B, A, D ��3�֣�
��3�����װ�õ������ԣ�1�֣�
��4����С��1�֣�
��5������ͨ������һ��ʱ�䣨1�֣�
��6��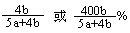 ��2�֣�
��2�֣�
���������������1����H�뷴Ӧ������ʵ����йأ������Ȼ�ѧ����ʽ�и����ʵ�ϵ�������릤H���Ӧ�����ϵ���ӱ�����HҲҪ�ӱ���
��2��ͼ�Т�װ�õ�����һ�Ǹ������壻����ʹSO2��O2���Ȼ�ϣ�����ѡB����װ�õ���������ȴSO3,ʹ����Һ�壬����ѡA,����װ�õ����ó�ȥβ��SO2������ѡD ��3�֣�
��3��һ��˵�������۲�������װ����ȡ���壬�ڳ���װ����װ���װ�뷴Ӧ��֮ǰ��������װ�õ������ԣ���ȷ��ʵ���˳�����С���1�֣�
��4����Ϊ2SO2��g��+O2��g�� 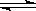 2SO3��g���Ƿ��ȷ�Ӧ�������¶ȣ��������ƶ�������ת���ʼ�С����1�֣�
2SO3��g���Ƿ��ȷ�Ӧ�������¶ȣ��������ƶ�������ת���ʼ�С����1�֣�
��5������ͨ������������ʹ������װ���е�SO2��SO3����������һ������������ȫ����NaOH���ա� ��1�֣�
��6��ʵ���������װ�â����ӵ�����Ϊ��Ӧ���ɵ�SO3��������,װ�â����ӵ�����Ϊĩ��Ӧ��SO2�����������Ը��ݻ�ѧ����ʽ���跴Ӧ��SO2����Ϊx��
2SO2��g��+O2��g�� 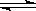 2SO3��g��
2SO3��g��
128 160
x b
x=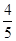 b
b
���Զ��������ת����Ϊ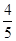 b/��a��
b/��a��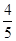 b��=
b��=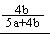 ��2�֣�
��2�֣�
���㣺���黯ѧʵ�顣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ��̽��ijЩ���������,�������ͼ��ʾ��ʵ��װ�á�ʵ��ʱ��A��D�в���������ͬʱͨ��C�С�(KΪֹˮ�У����ּг���������ȥ)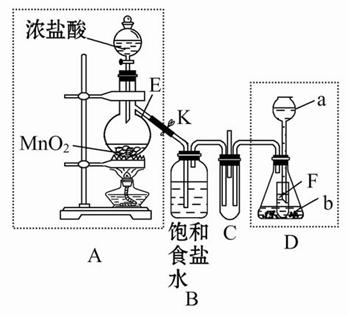
�ش��������⣺
(1)����ʵ��ǰ���A����װ�������Եķ����ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(2)����E�������ǣߣߣߣߣߣߣ���ƿ��С�Թ�F�������ǣߣߣߣߣߣߡ�
(3)��b����ʯ�ң�a�ǣߣߣߣߣߣ�ʱ����C���а��̲�����д�����ɰ��̵Ļ�ѧ����ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(4)��a����������Ϊ75%�����ᣬb���������Ʒ�ĩ����C��ʢ������BaCl2��Һʱ��д��C�з�����Ӧ�����ӷ���ʽ���ߣߣߣߣߣߣߣߣߣߣߣߡ�
(5)�ӻ����ĽǶȳ���������ʵ��װ������Ҫ�Ľ����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�á�
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���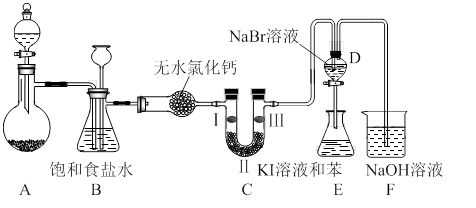
��1���Ʊ�����ѡ�õ�ҩƷΪ��������غ�Ũ���ᣬ��Ӧ�����ӷ���ʽΪ�� ��
��2��װ��B�������� �����ʵ�����ʱC�п��ܷ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ����ѡa��b��c��
| | a | b | c |
| I | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С����̽��SO2��Cl2�ܷ�����Ӧ���������ͼ��ʾ��ʵ��װ�ý���ʵ�顣
��1��װ��A�еķ�ӦΪ2KMnO4+16HCl(Ũ) ="=" 2KCl+2MnCl2+5Cl2��+8H2O����Ӧ�е��������� ������71 g Cl2ת�Ƶĵ����� mol��
װ��C������SO2����Ӧ�Ļ�ѧ����ʽ�� ��
��2��װ��B��պ��NaOH��Һ������������ ��
��3����A��C�з�Һ©���Ļ�����һ��ʱ���رջ���������Ӧ��ȫ��С��ͬѧ�ּ�������ʵ�顣
�ټ�ͬѧȡ����B����Һ���Թ��У������еμ�����AgNO3��Һ���а�ɫ�������ɡ���ͬѧ�ɴ���ΪSO2��Cl2�����˷�Ӧ�������Ǹ÷�Ӧ�������� �������ӷ��ţ���
����ͬѧ��Ϊ��ͬѧ�Ľ��۲���������ΪA�����ɵ�Cl2�л������ʣ�Ӧ����װ��A��B������һ��Ȼ���ٰ���ͬѧ�ķ������ɵõ���ȷ���ۡ�
��ͬѧ��ΪCl2�л��е������� ��ϴ��ƿ��ʢ���Լ��������� ��
�� ��ͬѧ����Ϊ�ס�����λͬѧ�Ľ��۾�����������ͬѧȡ����B����Һ���Թ��У������еμ�������ҺX���а�ɫ�������ɣ���ɵó����ۣ���SO2��Cl2ͬʱͨ��ˮ�У����Է�����Ӧ����ҺX�� ����ѡ����ţ���
A��BaCl2��Һ B��Ba (OH)2��Һ C��Ba (NO3)2��Һ D��Ʒ����Һ
SO2��Cl2ͬʱͨ��ˮ�з�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ƣ�NaNO2����¶�ڿ����л���������Ӧ���������ƣ�����ά��֯Ʒ��Ⱦɫ��Ư�ס����ࡢ��������ҩ�������й㷺Ӧ�ã�Ҳ���������ࡢ�����ʳƷ��Ⱦɫ�ͷ������������ж���������ʳƷ��ҵ�������ϸ����ơ�������ͼ��ʾ�������г�װ����ʡ�ԣ���ҩƷ��̽���������������ᷴӦ���������ɷ֡�
��֪����NO+NO2+2OH��==2NO2��+H2O ������Һ�����¶ȣ�NO2��21�棬NO��-152��
��1��Ϊ�˼���װ��A�����ɵ�����������������˳����������ӣ���A��C�� �� �� ��
��2����ӦǰӦ���ɼУ���ͨ��һ��ʱ�䵪�����ų�װ���еĿ�����Ŀ���� ��
��3���ڹرյ��ɼУ���Һ©������������70%�����A�в�������ɫ���塣
��ȷ��A�в��������庬��NO�����ݵ������� ��
��װ��E�������� ��
��4�������D��ͨ�����O2����װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ �����û��װ��C����ʵ�������ɵ�Ӱ���� ��
��5��ͨ������ʵ��̽�����̣��ɵó�װ��A�з�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣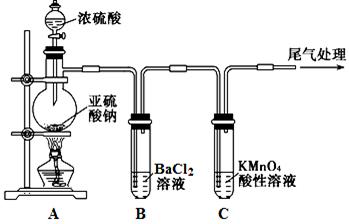
��֪��Na2SO3��H2SO4(Ũ)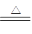 Na2SO4��SO2����H2O
Na2SO4��SO2����H2O
��ش��������⣺
��1��װ��A��ʢ��Ũ��������������� ��
��2��ʵ������У�C�е������� ��˵��SO2���� �ԡ�
��3��ʵ������У��۲쵽װ��B�г��������Եİ�ɫ������Ϊ̽���ð�ɫ�����ijɷ֣���С��ͬѧ����������ʵ�飺
����ʵ����ʵ�жϸð�ɫ�����ijɷ��� ���ѧʽ���������ð�ɫ������ԭ������� ������ĸ��ţ���
a��BaSO3�Ȳ�����ˮҲ��������
b��BaCl2��Һ�п����ܽ�������
c��BaCl2��Һ�п��ܻ���NaOH
d����A�Ƶõ�SO2�����п��ܻ�������
��4�������װ��A�е�ŨH2SO4����ŨHNO3���Դ�ʵ���Ƿ���Ӱ�첢������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧУ��ȤС���������װ�ý���ʵ��̽����a��bΪ���ɼУ����ȼ��̶�װ������ȥ����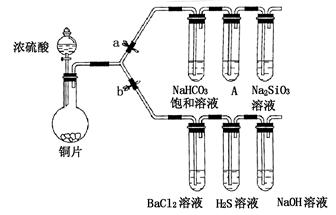
��1����֤̼����ǽ����Ե����ǿ��
������������ ����ҩƷ��a�ر�b��Ȼ�����Ũ���ᣬ���ȣ�
��װ��A�е��Լ��� ��
����˵��̼�ķǽ����Աȹ�ǿ��ʵ�������� ��
��2����֤SO2�������ԡ���ԭ�Ժ������������ͨ��
�ٴ�b���ر�a������֤SO2���������ԵĻ�ѧ����ʽ�� ��
����������SO2ͨ��NaOH��Һ�У������ӷ���ʽ�� ��
��BaCl2��Һ������������ֳ����ݣ��ֱ�μ�������Һ���������ij����Ļ�ѧʽ�����±���Ӧλ�á�
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
��֪��Na2SO3��H2SO4(Ũ) Na2SO4��SO2����H2O
Na2SO4��SO2����H2O
��ش��������⣺
��1��װ��A��ʢ��Ũ��������������� ��
��2��ʵ������У�C�е������� ��˵��SO2���� �ԡ�
��3��ʵ������У��۲쵽װ��B�г��������Եİ�ɫ������Ϊ̽���ð�ɫ�����ijɷ֣���С��ͬѧ����������ʵ�飺
����ʵ����ʵ�жϸð�ɫ�����ijɷ��� ���ѧʽ���������ð�ɫ������ԭ������� ������ĸ��ţ���
a��BaSO3�Ȳ�����ˮҲ��������
b��BaCl2��Һ�п����ܽ�������
c��BaCl2��Һ�п��ܻ���NaOH
d����A�Ƶõ�SO2�����п��ܻ�������
��4��д��B�в�����ɫ���������ӷ���ʽΪ��____________________________
��5�������װ��A�е�ŨH2SO4����ŨHNO3���Դ�ʵ���Ƿ���Ӱ�첢������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С��Ϊ����֤SO2��Cl2��Ư���ԣ����������ͼ��ʾ��ʵ��װ�á���ش��������⣺
��1�������Ʊ�SO2��Cl2�����ݵ�ԭ���ֱ��ǣ�Na2SO3+H2SO4=Na2SO4+H2O+SO2����MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O������ͼA��Eװ����������Cl2��װ����
MnCl2+Cl2��+2H2O������ͼA��Eװ����������Cl2��װ����
������ţ�����Ӧ�����������ֵ������� ��
��2����Ӧ��ʼ����B��D�Թ��е�Ʒ����Һ����ɫ��ֹͣͨ����B��D�����Թ��е���Һ���ȣ�B�Թ��е������� ��
��3��װ��C�������� ��
��4��NaOH����������Һ�ֱ����������巴Ӧ�����ӷ���ʽ�� �� ��
��5����С��ͬѧ�����������Ϻ�ͨ��Ʒ����Һ��һ��ʱ���Ʒ����Һ��������ɫ���������ϵ�֪���������尴�����1��1��ϣ�����ˮ��Ӧ���������ֳ����ᣬ���ʧȥƯ�����á��÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com