
ЁОЬтФПЁПЭъГЩЯТСаЬюПе
(1) 2-фхБћЭщЕФЯћШЅЗДгІ(ЛЏбЇЗНГЬЪН)______________________________________ЃЛ
(2) 1,2-ЖўфхввЭщЕФЫЎНт(ЛЏбЇЗНГЬЪН)_______________________________________ЃЛЁЏ
(3)ЖўЧтЯуЖЙЫи( )ГЃгУзїЯуЖЙЫиЕФЬцДњЦЗЃЌМјБ№ЖўЧтЯуЖЙЫиКЭЫќЕФвЛжжЭЌЗжвьЙЙЬх(
)ГЃгУзїЯуЖЙЫиЕФЬцДњЦЗЃЌМјБ№ЖўЧтЯуЖЙЫиКЭЫќЕФвЛжжЭЌЗжвьЙЙЬх( )ашвЊгУЕНЕФЪдМСгаЃКNaOHШмвКЁЂ_________________ЃЛ
)ашвЊгУЕНЕФЪдМСгаЃКNaOHШмвКЁЂ_________________ЃЛ
(4)ФГЬўРрЛЏКЯЮяAЕФжЪЦзЭМБэУїЦфЯрЖдЗжзгжЪСПЮЊ84ЃЌКьЭтЙтЦзБэУїЗжзгжаКЌгаЬМЬМЫЋМќЃЌКЫДХЙВеёЧтЦзБэУїЗжзгжажЛгавЛжжРраЭЕФЧтдзгЁЃдђAЕФНсЙЙМђЪНЮЊ______________________ЃЛAЪЧЗёДцдкЫГЗДвьЙЙЬхЃП________ (ЬюЁАЪЧЁБЛђепЁАЗёЁБ)ЁЃ
ЁОД№АИЁП CH3-CHBr-CH3+NaOH![]() CH3-CH=CH2Ёќ+NaBr+H2O CH2BrCH2Br+2NaOH
CH3-CH=CH2Ёќ+NaBr+H2O CH2BrCH2Br+2NaOH![]() CH2OHCH2OH+2NaBr ЯЁСђЫсЃЌТШЛЏЬњЛђХЈфхЫЎ
CH2OHCH2OH+2NaBr ЯЁСђЫсЃЌТШЛЏЬњЛђХЈфхЫЎ ![]() Зё
Зё
ЁОНтЮіЁПБОЬтжївЊПМВщТБДњЬўЕШгаЛњЮяЕФаджЪЁЃ
(1) 2-фхБћЭщЕФЯћШЅЗДгІЕФЛЏбЇЗНГЬЪНЃКCH3-CHBr-CH3+NaOH![]() CH3-CH=CH2Ёќ+NaBr+H2OЁЃ
CH3-CH=CH2Ёќ+NaBr+H2OЁЃ
(2) 1,2-ЖўфхввЭщЕФЫЎНтЗДгІЕФЛЏбЇЗНГЬЪНЃКCH2BrCH2Br+2NaOH![]() CH2OHCH2OH+2NaBrЁЃ
CH2OHCH2OH+2NaBrЁЃ
(3)ЖўЧтЯуЖЙЫи(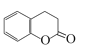 )ЫЎНтВњЩњЗгєЧЛљЃЌЫќЕФвЛжжЭЌЗжвьЙЙЬх(
)ЫЎНтВњЩњЗгєЧЛљЃЌЫќЕФвЛжжЭЌЗжвьЙЙЬх(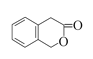 )ЫЎНтВњЩњДМєЧЛљЃЌПЩвдЪЙЖўепдкЧтбѕЛЏФЦШмвКзїгУЯТЫЎНтЃЌдйРћгУЯЁСђЫсЛжИДЫсаддзгЭХЃЌзюКѓЪЙгУТШЛЏЬњШмвКЛђХЈфхЫЎМгвдМјБ№ЃЌШмвКГЪЯжзЯЩЋЛђВњЩњАзЩЋГСЕэЕФЪЧЖўЧтЯуЖЙЫиЁЃМјБ№ЪдМСгаЃКNaOHШмвКЁЂЯЁСђЫсЁЂТШЛЏЬњШмвКЛђХЈфхЫЎЁЃ
)ЫЎНтВњЩњДМєЧЛљЃЌПЩвдЪЙЖўепдкЧтбѕЛЏФЦШмвКзїгУЯТЫЎНтЃЌдйРћгУЯЁСђЫсЛжИДЫсаддзгЭХЃЌзюКѓЪЙгУТШЛЏЬњШмвКЛђХЈфхЫЎМгвдМјБ№ЃЌШмвКГЪЯжзЯЩЋЛђВњЩњАзЩЋГСЕэЕФЪЧЖўЧтЯуЖЙЫиЁЃМјБ№ЪдМСгаЃКNaOHШмвКЁЂЯЁСђЫсЁЂТШЛЏЬњШмвКЛђХЈфхЫЎЁЃ
(4)ФГЬўРрЛЏКЯЮяAЕФжЪЦзЭМБэУїЦфЯрЖдЗжзгжЪСПЮЊ84ЃЌКьЭтЙтЦзБэУїЗжзгжаКЌгаЬМЬМЫЋМќЃЌЫљвдAЕФЗжзгЪНЮЊC6H12ЃЌКЫДХЙВеёЧтЦзБэУїЗжзгжажЛгавЛжжРраЭЕФЧтдзгЁЃдђAЕФНсЙЙМђЪНЮЊ![]() ЃЛAЕФЫЋМќЬМдзгЫљСЌГЩЗжЭъШЋЯрЭЌЃЌЫљвдAВЛДцдкЫГЗДвьЙЙЬхЁЃ
ЃЛAЕФЫЋМќЬМдзгЫљСЌГЩЗжЭъШЋЯрЭЌЃЌЫљвдAВЛДцдкЫГЗДвьЙЙЬхЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЗДгІПЩгУРызгЗНГЬЪНЁАH++OH-=H2OЁББэЪОЕФЪЧЃЈ ЃЉ
A.NaHSO4ШмвКгыBa(OH)2ШмвКЛьКЯ
B.NH4ClШмвКгыCa(OH)2ШмвКЛьКЯ
C.HNO3ШмвКгыKOHШмвКЛьКЯ
D.CH3COOHШмвКгыNaOHШмвКЛьКЯ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬьШЛЦјМШЪЧИпаЇНрОЛЕФФмдДЃЌвВЪЧживЊЕФЛЏЙЄдСЯЁЃ
(1)МзЭщЗжзгЕФНсЙЙЪНЮЊ_________ЃЌПеМфЙЙаЭЮЊ_______________аЮЁЃ
(2)вбжЊ25ЁцЁЂ101kPa ЪБЃЌ1 gМзЭщЭъШЋШМЩеЩњГЩвКЬЌЫЎЗХГі55.64 kJШШСПЃЌдђИУЬѕМўЯТЗДгІCH4(g)+2O2(g)ЃНCO2(g)+2H2O(l)ЕФЁїHЃН____________kJЁЄmol-1ЁЃ
(3)МзЭщИпЮТЗжНтЩњГЩЧтЦјКЭЬПКкЁЃдкУмБеШнЦїжаНјааДЫЗДгІЪБвЊЭЈШыЪЪСППеЦјЪЙВПЗжМзЭщШМЩеЃЌЦфФПЕФЪЧ__________________________________________ЁЃ
(4)ШМСЯЕчГиЕФЙЄзїдРэЪЧНЋШМСЯКЭбѕЛЏМС(ШчO2)ЗДгІЫљВњЩњЕФФмСПжБНгзЊЛЏЮЊЕчФмЁЃгУМзЭщ-ПеЦјМюад(KOHШмвК)ШМСЯЕчГизїЕчдДЃЌЕчНтCuCl2ШмвКЁЃвбжЊМзЭщПеЦјМюадШМСЯЕчГиЕФзмЗДгІЮЊЃКCH4+2O2+2KOHЃНK2CO3+3H2OЃЌзАжУШчЯТЭМЫљЪОЃК

ЂйaЕчМЋУћГЦЮЊ____________ЁЃ
ЂкcЕчМЋЕФЕчМЋЗДгІЪНЮЊ_________________________________ЁЃ
ЂлМйЩшCuCl2ШмвКзуСПЃЌЕБФГЕчМЋЩЯЮіГі3.2 g Н№ЪєCuЪБЃЌРэТлЩЯШМСЯЕчГиЯћКФЕФМзЭщдкБъзМзДПіЯТЕФЬхЛ§ЪЧ__________LЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЖдЪЕбщвЧЦїЬиЕуЕФУшЪіе§ШЗЕФЛђЪЕбщФмДяЕНдЄЦкФПЕФЕФЪЧ(ЁЁЁЁ)
A.ЭаХЬЬьЦНБъГпЕФЁА0ЁБПЬЖШдкжаМф
B.СПЭВЕФЁА0ЁБПЬЖШдкЯТУц
C.гУ10 mLСПЭВШЅСПШЁ7.50 mLЯЁбЮЫс
D.гУЭаХЬЬьЦНГЦСП25.2 g NaClОЇЬх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАДШчЭМзАжУЃЌГжајЭЈШыXЦјЬхЃЌПЩвдПДЕНaДІгаКьЩЋЮяжЪЩњГЩЃЌbДІБфРЖЃЌcДІЕУЕНвКЬхЃЌдђXЦјЬхЪЧ

A. H2 B. CH3CH2OH(Цј) C. CO2 D. COКЭH2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЪЕбщаЁзщбаОПШмвКжаAgNO3КЭNa2SЕФЗДгІЁЃ
ЪЕбщ | ЪдМС | ЯжЯѓ | |
| ЪдЙм | ЕЮЙм | |
ЃЈpH = 4ЃЉ |
ЃЈpH = 9ЃЉ | ГіЯжКкЩЋГСЕэ | |
ЃЈ1ЃЉгУРызгЗНГЬЪННтЪЭNa2SШмвКpH > 7ЕФдвђЃК________ЁЃ
ЃЈ2ЃЉЪЕбщаЁзщЭЌбЇШЯЮЊКкЩЋГСЕэжаПЩФмКЌгаAg2OЁЂAg2SЛђAgЃЌЩшМЦЪЕбщбщжЄЁЃ
вбжЊЃКiЃЎХЈЯѕЫсФмНЋAg2SзЊЛЏЮЊ![]() КЭ
КЭ![]() ЃЛ
ЃЛ
iiЃЎAg2OФмШмНтдкХЈАБЫЎжааЮГЩвјАБШмвКЃЌЖјAg2SКЭAgОљВЛФмЁЃ
Ђй ЩшМЦВЂЪЕЪЉШчЯТЪЕбщЃЌжЄЪЕГСЕэжаКЌгаAg2SЁЃ

ЪдМС1КЭЪдМС2ЗжБ№ЪЧ_________ЁЂ_________ЁЃ
ЯжЯѓ1КЭЯжЯѓ2ЗжБ№ЪЧ_________ЁЂ_________ЁЃ
Ђк ЩшМЦВЂЪЕЪЉШчЯТЪЕбщЃЌжЄЪЕГСЕэжаВЛКЌгаAg2OЃЌНЋЪЕбщВйзїКЭЯжЯѓВЙГфЭъећЁЃ
ЪЕбщВйзї | ЪЕбщЯжЯѓ | |
ВНжшi | ШЁЩйСПвјАБШмвКЃЌЯђЦфжаЕЮМгбЮЫс | ГіЯжАзЩЋГСЕэ |
ВНжшii | ШЁЩйСПЯДЕгКѓЕФКкЩЋГСЕэЃЌ____________ | ____________ |
Ђл ОМьбщЃЌГСЕэВЛКЌгаAgЁЃ

ЃЈ3ЃЉЪЕбщаЁзщЭЌбЇШЯЮЊAgNO3ШмвКОпгабѕЛЏадЃЌдквЛЖЈЬѕМўЯТФмЙЛбѕЛЏNa2SЃЌЩшМЦЪЕбщНјаабаОПЃЈЪЕбщзАжУШчгвЭМЫљЪОЃЉЃЌВтЕУЕчбЙЮЊaЃЈ![]() ЃЉЁЃ
ЃЉЁЃ
ЖдAgNO3ШмвКжабѕЛЏ![]() ЕФЮяжЪНјааЭЦВтЃК
ЕФЮяжЪНјааЭЦВтЃК
МйЩш1ЃК ![]() ЕФAgNO3ШмвКжа
ЕФAgNO3ШмвКжа![]() бѕЛЏСЫ
бѕЛЏСЫ![]() ЃЛ
ЃЛ
МйЩш2ЃК ![]() ЕФAgNO3ШмвКжа
ЕФAgNO3ШмвКжа![]() бѕЛЏСЫ
бѕЛЏСЫ![]() ЁЃ
ЁЃ
РћгУгвЭМзАжУМЬајбаОПЃЈвбжЊЃКЕчбЙДѓаЁЗДгГСЫЮяжЪбѕЛЏЛЙдадЧПШѕЕФВювьЃЛЮяжЪбѕЛЏадгыЛЙдадЧПШѕВювьдНДѓЃЌЕчбЙдНДѓЃЉЁЃ
Ђй НЋ![]() ЕФAgNO3ШмвКЬцЛЛЮЊ_______ШмвКЃЌМЧТМЕчбЙЮЊbЃЈ
ЕФAgNO3ШмвКЬцЛЛЮЊ_______ШмвКЃЌМЧТМЕчбЙЮЊbЃЈ![]() ЃЉЁЃ
ЃЉЁЃ
Ђк ЩЯЪіЪЕбщжЄЪЕСЫбѕЛЏ![]() ЕФЮяжЪжавЛЖЈАќКЌ
ЕФЮяжЪжавЛЖЈАќКЌ![]() ЃЌЦфжЄОнЪЧ______ЁЃ
ЃЌЦфжЄОнЪЧ______ЁЃ
ЪЕбщНсТлЃКAgNO3ШмвКгыNa2SШмвКЕФЗДгІРраЭгыЗДгІЬѕМўгаЙиЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЬжмЦкжїзхдЊЫиXЁЂYЁЂZЁЂWЕФдзгађЪ§вРДЮдіДѓЁЃЫФжждЊЫиаЮГЩЕФЕЅжЪвРДЮЮЊmЁЂnЁЂpЁЂqЃЛетаЉдЊЫизщГЩЕФЖўдЊЛЏКЯЮяrЁЂtЁЂuЃЌЦфжаuФмЪЙЦЗКьШмвКЭЫЩЋЃЌvЕФЫзУћНаЩеМюЁЃЩЯЪіЮяжЪЕФзЊЛЏЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ( )

A. дзгАыОЖЕФДѓаЁЃКW>Z>Y>X
B. tгыrЗДгІЪБЃЌrЮЊбѕЛЏМС
C. ЩњЛюжаПЩгУuЪЙЪГЮядіАз
D. ZЗжБ№гыYЁЂWзщГЩЕФЛЏКЯЮяжаЛЏбЇНЁРраЭПЩФмЯрЭЌ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПИпЬњЕчГиОпгаБШФмСПИпЁЂЮоЮлШОЕФЬиЕуЃЌгУЯТЭМФЃФтЦфЙЄзїдРэЃЈЗХЕчЪБСНЕчМЋОљгаЮШЖЈЕФН№ЪєЧтбѕЛЏЮяЩњГЩЃЉЃЌЯТСагаЙиЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ

A. ЗХЕчЪБЃЌЕчзггЩе§МЋЭЈЙ§ЭтЕчТЗСїЯђИКМЋ
B. ЗХЕчЪБЃЌИКМЋЩЯЕФЕчМЋЗДгІЪНЮЊЃКZn-2e ЉЄ+2H2O = Zn(OH)2+2H+
C. ГфЕчЪБЃЌвѕМЋЧјШмвКЕФpHМѕаЁ
D. ГфЕчЪБЃЌбєМЋЩЯЕФЕчМЋЗДгІЪНЮЊЃКFe(OH)3-3e ЉЄ+5OH ЉЄ = FeO42 ЉЄ+4H2O
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПаЁУїАДЯТЭМзАКУСЫЪЕбщзАжУ(СННКЭЗЕЮЙмжаЕФЙ§бѕЛЏЧтШмвКЬхЛ§ЯрЕШЃЌХЈЖШЗжБ№ЮЊ5%КЭ10%)ЃЌЪЕбщЪБЃЌЭЌЪБЭъШЋФѓБтСНЕЮЙмЕФНКЭЗЃЌВЂЙлВьЪЕбщЯжЯѓЁЃ

ЃЈ1ЃЉаЁУїЕФЪЕбщФПЕФЪЧЃК__________________________ЁЃ
ЃЈ2ЃЉзАжУжаГЄВЃСЇЕМЙмЕФзїгУЪЧЃК_________________ЃЛКьФЋЫЎЕФзїгУЪЧ________ЁЃ
ЃЈ3ЃЉФуЙРМЦСНИљВЃСЇЕМЙмжаЕФЪЕбщЯжЯѓЪЧ_____________ЃЛРэгЩЪЧ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com