
����Ŀ�������£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2 (g) �� 3 H2(g) ![]() 2NH3(g)��
2NH3(g)��
��1������Ӧijʱ��tʱ��n t (N2) = 13 mol��n t (NH3) = 6 mol����a =____mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������NH3�ĺ���(�������)Ϊ25%��ƽ��ʱNH3�����ʵ���_____��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȡ���ͬ����n(ʼ)��n(ƽ) =______��
��4��ԭ��������У�a��b =_____��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ���(N2)�æ�(H2)= ______��
��6��ƽ���������У�n(N2)��n(H2)��n(NH3) =______��
���𰸡�16 mol 8 mol 5��4 2��3 1��2 3��3��2
��������
��1�����ݻ�ѧ����ʽ����aֵ��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������������ʵ�����![]() 32mol������NH3�ĺ���(�������)Ϊ25%���㰱�������ʵ�����
32mol������NH3�ĺ���(�������)Ϊ25%���㰱�������ʵ�����
��3�����ò���������ԭ�����������ʵ�����
��4�����ݣ�1����֪a=16�����ݣ�3����֪ԭ�����������ʵ�����40mol��
��5�����á�����ʽ������N2��H2��ת����֮�ȣ�
��6�����ݡ�����ʽ���ж�ƽ���������и���������ʵ����ȣ�
��1������N2 (g)�� 3H2(g)![]() 2NH3(g)��Ӧ����֪����Ӧijʱ��tʱ��n (NH3) = 6 mol��������n (N2) =3 mol����ʼ����n (N2)= 13mol+ 3mol =16mol��
2NH3(g)��Ӧ����֪����Ӧijʱ��tʱ��n (NH3) = 6 mol��������n (N2) =3 mol����ʼ����n (N2)= 13mol+ 3mol =16mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������������ʵ�����![]() 32mol��NH3�ĺ���(�������)Ϊ25%�������������ʵ�����32mol��25%=8mol��
32mol��NH3�ĺ���(�������)Ϊ25%�������������ʵ�����32mol��25%=8mol��
��3���跴Ӧ���������ʵ�������n��
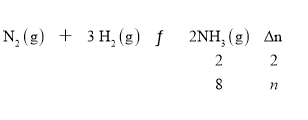
n=![]() 8mol������ԭ�����������ʵ�����32mol+8mol=40mol��ԭ���������ƽ��������������ʵ���֮��40:32=5:4��
8mol������ԭ�����������ʵ�����32mol+8mol=40mol��ԭ���������ƽ��������������ʵ���֮��40:32=5:4��
��4�����ݣ�1����֪a=16�����ݣ�3����֪ԭ�����������ʵ�����40mol������b=40mol-16mol=24mol��a��b =16:24=2:3��
��5��
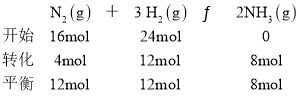
N2��ת����Ϊ![]() ��H2��ת����Ϊ
��H2��ת����Ϊ![]() ��������(N2)����(H2)=1:2��
��������(N2)����(H2)=1:2��
��5������
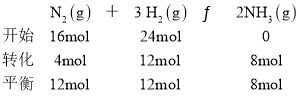
ƽ���������У�n(N2)��n(H2)��n(NH3) =12:12:8=3:3:2��


 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�������£�0.01 mol/L MOH��Һ��pHΪ10��MOH(aq)��H2SO4(aq)��Ӧ����1 mol���ε���H����24.2 kJ��mol��1��ǿ����ǿ���ϡ��Һ���к���Ϊ��H����57.3 kJ��mol��1����MOH��ˮ��Һ�е������HΪ( )
A. ��69.4 kJ��mol��1 B. ��45.2 kJ��mol��1
C. ��69.4 kJ��mol��1 D. ��45.2 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ����0.50mol/LNaCl��Һ480mL����ʹ��NaCl�������ƣ������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ����ȷ��0.1 g����ҩ�ס��ձ�����������______��_______�Լ�����������ƬֽƬ��
��2�����㡣���Ƹ���Һ��ȡNaCl����_______g��
��3�����ù��̡�
����ƽ���㡣
�ڳ���������NaCl����Ӧ������ƽ��_______��������������������������
�۳�����ϣ���ҩƷ�����ձ��С�
���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����________��
��ת�ơ�ϴ�ӡ���ת��ʱӦʹ��_____��������Ҫϴ���ձ�2-3����Ϊ��______��
���ݡ�ҡ�ȡ�
�߽���õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�������ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ�ȡ�
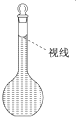
��4�������ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�______����ߡ�����ƫ�͡�����Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ϡ����ķ�Ӧ�У���֪10sĩ�����Ũ�ȼ�����0.6 mol/L�������Ƿ�Ӧ��������Һ����ı仯����10s��������������ƽ����Ӧ������( )
A. 0.02 mol/(Lmin)B. 1.2 mol/(Lmin)
C. 1.8mol/(Lmin)D. 0.18 mol/(Lmin)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ʊ������ԭ��Ϊ��NaCl+CO2+NH3+H2O=NH4Cl+ NaHCO3�������������գ�
(1)������Ӧ��ϵ�г��ֵļ��ֶ�����Ԫ�أ��ǽ�������ǿ����_____���ڶ�����ԭ�Ӱ뾶�ɴ�С����______��
(2)��Ӧ��ϵ�г��ֵķǽ���Ԫ�ؿ��γɶ��ֻ����� �����к�笠����ӿռ乹����ͬ�������л���ĵ���ʽ��______���÷���Ϊ_____( ѡ���������������Ǽ�����)���ӡ�
(3)д������Ԫ����������δ�ɶԵ��ӵ�ԭ�Ӻ�������Ų�ʽ_____�����й��ڸ�Ԫ�غ���Ԫ��֮��ǽ����Դ�С�ж�������ȷ����____(����)
a.����������Ӧˮ��������� b.��Ԫ���γɻ�����Ļ��ϼ�
c.��̬�⻯����ȶ��� d. �⻯��ˮ��Һ�������
(4)����������ľ���ҲӦ�������ڽ��ʯ����ʵ�ʽ�Ϊ���ӣ���������Ϊ��������������������Ժͷ����������ѱ��ϸ�ִ�С����磺�����ױ�����������ÿ����ԭ�Ӷ��γ��������ÿ��NH3����Χ______��NH3ͨ��������ϡ�
(5)������ FeF3�۵����1000������Fe(CO)5 ���۵�ȴ���� 0����FeF3�۵�Զ����Fe(CO)5��ԭ�������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС��Ϊ�˲ⶨijƷ�����Ͻ������ĺ����������������ʵ�飺
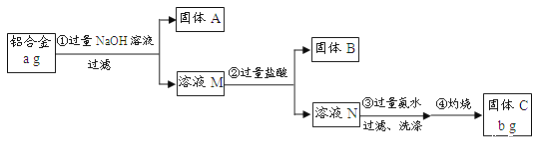
��֪�������Ͻ����Ҫ�ɷ�ΪAl2O3��MgO��CuO��SiO2��
��ش��������⣺
��1������A�ijɷ���_______��
��2�����ɹ���B�Ļ�ѧ����ʽΪ_____������������ɳ��������ӷ���ʽΪ_____��
��3�����鲽����г����Ƿ�ϴ�Ӹɾ���ʵ�����Ϊ______��
��4������Ʒ����������������______������a��b��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�飬����ȷ�����䷴Ӧ�����ӷ���ʽ��
A.��Na2SO3��Һ��������Cl2��3![]() +Cl2+H2O = 2
+Cl2+H2O = 2![]() +2
+2![]() +
+![]()
B.��CaCl2��Һ��ͨ��CO2��Ca2++H2O+CO2=CaCO3��+2H+
C.��H2O2��Һ�еμ�����FeCl3��2Fe3++H2O2=O2��+2H++2Fe2+
D.ͬŨ��ͬ���NH4HSO4��Һ��NaOH��Һ��ϣ�![]() +OH��=NH3��H2O
+OH��=NH3��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����в���ǰ36��Ԫ�ص����ʻ�ԭ�ӽṹ���±�
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
R | ��̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2������ |
S | ��������ˮ���ҷ�Ӧ��������Һ�������� |
T | ��̬ԭ��3d�������1������ |
X | �� |
��1��RԪ�صĵ�һ������Ҫ������ͬ�������ڵ�Ԫ�أ�ԭ����________________________________________________________��
��2��SԪ�صĻ��ϼ��Ƿ������ۣ�__________��ԭ����__________________________________�����������Ų�ʽΪ________________________��
��3��TԪ�ص�ԭ��N�ܲ��ϵ�����Ϊ__________����ԭ�ӽṹʾ��ͼΪ__________��
��4��X�ĺ�������Ų�ͼΥ����__________����X���ʡ�������μ����������εȿ����������ȼ��ʱ�����������ɫ�Ĺ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ��____________________________________________________________________��
���𰸡� ��ԭ��2p���������������ͣ��ȶ� �� F�ĵ縺�����ֻ�ܵõ��� 2s22p5 2  �������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�����������������RԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ�R��NԪ�أ�SԪ�صĵ�������ˮ���ҷ�Ӧ��������Һ�������ԣ�S��FԪ�أ�TԪ�صĻ�̬ԭ��3d�������1�����ӣ�T��21��Ԫ��Sc�� XԪ�ص�ԭ�Ӻ�����12�����ӣ�X��MgԪ�ء�
�������������Ϸ�������1��R��NԪ������ԭ��2p���������������ͣ��ȶ�,���Ե�һ������Ҫ������ͬ�������ڵ�OԪ����
��2��Ԫ��F�ĵ縺�����ֻ�ܵõ���������FԪ��û�����ۣ�FԪ�ص����������Ų�ʽΪ2s22p5��
��3��Scԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p63d14s2������N�ܲ��ϵ�����Ϊ2����ԭ�ӽṹʾ��ͼΪ ����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
�����͡�������
��������
20
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | ��������ˮ���ҷ�Ӧ��������Һ�������� |
X | L��p��������s��������2�� |
Y | ��������Ԫ�صļ������а뾶��С |
Z | L��������δ�ɶԵ��� |
��1��д��Ԫ��X�����ӽṹʾ��ͼ__________��
��2��д��YԪ������������ˮ����ֱ���HCl��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________��_________________________��
��3��д��Z��Y�ĵ����Ų�ʽ______________��________________��
��4��Ԫ��T����Ԫ����ȣ��ǽ����Խ�ǿ����__________(��Ԫ�ط��ű�ʾ)�����б�������֤����һ��ʵ����__________��
A����̬�⻯��Ļӷ��Ժ��ȶ���
B�����ʷ����еļ���
C����Ԫ�صĵ縺��
D�������������
E���⻯����X��H���ļ���(X����T��Cl����Ԫ��)
F������������Ȼ���еĴ�����ʽ
��5��̽Ѱ���ʵ����ʲ�������ѧϰ����Ҫ����֮һ��T��X��Y��Z����Ԫ�صĵ����л�ѧ�������Բ�ͬ���������ֵ��ʵ���__________(��Ԫ�ط���)��������________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ�����������ͬ���칹�塣��ѧ�о����������ַ��Ӷ����п������ԡ�
(1)��ष����Ǵ����ƽ�����壬��ṹ��ʽ��ͼ��ʾ![]() ����ԭ�ӵ��ӻ������ʽ��_____����ष����У���Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____����ष����к���_____���� ����
����ԭ�ӵ��ӻ������ʽ��_____����ष����У���Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____����ष����к���_____���� ����
(2)���ȶ���ऺϲ������д��ڵ�������������_____������ţ���
a�����Ӽ� b����λ�� c�������� d���Ǽ��Լ� e�����
(3)���ȶ���ऺϲ������У�Pt2+����λ���� 4�����������ӻ���ʽ������ sp3���������ɣ�_____________��
(4)����һ�ֶ��ȶ���ऺϲ����ӽṹ��ͼ��ʾ���÷�����_____���ӣ�ѡ���������������Ǽ���������
![]()
(5)CO(NH2)2 ������ˮ������Ҫԭ����_________________________��
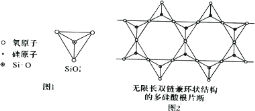
(6)Si Ԫ���� Si��O��Si �����ɿ���磬�����������壨ͼ l�����ӳ������ĵ�����˫����ͼ 2���ṹ��ͼ 2 ��ʾ�Ķ��������ӵĻ�ѧʽͨʽΪ_____���Ժ�������n �Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com