
����Ŀ�����ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ�����������ͬ���칹�塣��ѧ�о����������ַ��Ӷ����п������ԡ�
(1)��ष����Ǵ����ƽ�����壬��ṹ��ʽ��ͼ��ʾ![]() ����ԭ�ӵ��ӻ������ʽ��_____����ष����У���Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____����ष����к���_____���� ����
����ԭ�ӵ��ӻ������ʽ��_____����ष����У���Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____����ष����к���_____���� ����
(2)���ȶ���ऺϲ������д��ڵ�������������_____������ţ���
a�����Ӽ� b����λ�� c�������� d���Ǽ��Լ� e�����
(3)���ȶ���ऺϲ������У�Pt2+����λ���� 4�����������ӻ���ʽ������ sp3���������ɣ�_____________��
(4)����һ�ֶ��ȶ���ऺϲ����ӽṹ��ͼ��ʾ���÷�����_____���ӣ�ѡ���������������Ǽ���������
![]()
(5)CO(NH2)2 ������ˮ������Ҫԭ����_________________________��
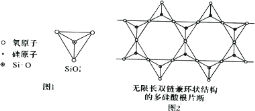
(6)Si Ԫ���� Si��O��Si �����ɿ���磬�����������壨ͼ l�����ӳ������ĵ�����˫����ͼ 2���ṹ��ͼ 2 ��ʾ�Ķ��������ӵĻ�ѧʽͨʽΪ_____���Ժ�������n �Ĵ���ʽ��ʾ����
���𰸡�sp2 N��C��H 11 bd ��Pt2+�� sp3 �ӻ����������λ������ȶ���ऺϲ�Ϊ������ṹ��������˳���칹�� �Ǽ��� CO(NH2)2 ��ˮ����֮���γ���� (Si4O11)n6n-
��������
(1)�ӽṹ��ʽ![]() ���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3���ɴ˿ɵó��ӻ������ʽ����ष����У���N��C��H����Ԫ�أ��ɷǽ����Կ�ȷ����Ԫ�صĵ縺���ɴ�С��˳����ष����У�����ԭ�Ӽ�ֻ���γ�1���� �����ɴ˿ɼ��������� ������Ŀ��
���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3���ɴ˿ɵó��ӻ������ʽ����ष����У���N��C��H����Ԫ�أ��ɷǽ����Կ�ȷ����Ԫ�صĵ縺���ɴ�С��˳����ष����У�����ԭ�Ӽ�ֻ���γ�1���� �����ɴ˿ɼ��������� ������Ŀ��
(2)���ȶ���ऺϲ������У������ܺ������Ӽ��������������������ԭ��������������λ������N5-�д��ڷǼ��Լ���
(3)���ȶ���ऺϲ������У�Pt2+����λ���� 4��������ӻ���ʽ��sp3�����ܴ���˳���칹��
(4)����һ�ֶ��ȶ���ऺϲ����ӽṹ��ͼ��ʾ���÷��ӽṹ�Գơ�
(5)CO(NH2)2���γɷ��Ӽ�����������������ˮ��
(6)ȡͼ�нṹ��Ԫ��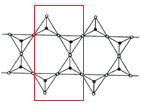 ���þ�̯���������������ӵĻ�ѧʽͨʽ��
���þ�̯���������������ӵĻ�ѧʽͨʽ��
(1)�ӽṹ��ʽ![]() ���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3����ԭ�ӵ��ӻ������ʽΪsp2����ष����У���N��C��H����Ԫ�أ��ǽ�����N>C>H�����Ԫ�صĵ縺���ɴ�С��˳��ΪN>C>H����ष����У�����ԭ�Ӽ�ֻ���γ�1���� ��������ष����к���4��̼̼�� ����2��̼���� ����5��̼���� ���������� ������ĿΪ11����Ϊ��sp2��N>C>H��11��
���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3����ԭ�ӵ��ӻ������ʽΪsp2����ष����У���N��C��H����Ԫ�أ��ǽ�����N>C>H�����Ԫ�صĵ縺���ɴ�С��˳��ΪN>C>H����ष����У�����ԭ�Ӽ�ֻ���γ�1���� ��������ष����к���4��̼̼�� ����2��̼���� ����5��̼���� ���������� ������ĿΪ11����Ϊ��sp2��N>C>H��11��
(2)���ȶ���ऺϲ������У������ܺ������Ӽ��������������������ԭ��������������λ������N5-�д��ڷǼ��Լ�����ѡbd����Ϊ��bd��
(3)���ȶ���ऺϲ������У�Pt2+����λ���� 4��������ӻ���ʽ��sp3�����ܴ���˳���칹������Ϊ��Pt2+�� sp3 �ӻ����������λ������ȶ���ऺϲ�Ϊ������ṹ��������˳���칹�塣��Ϊ����Pt2+��sp3 �ӻ����������λ������ȶ���ऺϲ�Ϊ������ṹ��������˳���칹�壻
(4)�ɶ��ȶ���ऺϲ����ӽṹ���Կ������÷��ӽṹ�Գƣ�Ϊ�Ǽ��Է��ӡ���Ϊ���Ǽ��ԣ�
(5)CO(NH2)2����Ӽ���γ����������������ˮ����ԭ��ΪCO(NH2)2 ��ˮ����֮���γ��������Ϊ��CO(NH2)2 ��ˮ����֮���γ������
(6)�����������幹�ɣ�ÿ����ԭ����4����ԭ�ӹ��������壬ȡͼ�нṹ��Ԫ�� ���ṹ��Ԫ�д��ڹ��ñ��ϵ�Oԭ��Ϊÿ���ṹ��Ԫ�ṩ1/2�����ڽṹ��Ԫ�ڵ�Oԭ����9��������4����ͶӰ��Si�غϣ��ʽṹ��Ԫ�й���Oԭ����ĿΪ9+4��
���ṹ��Ԫ�д��ڹ��ñ��ϵ�Oԭ��Ϊÿ���ṹ��Ԫ�ṩ1/2�����ڽṹ��Ԫ�ڵ�Oԭ����9��������4����ͶӰ��Si�غϣ��ʽṹ��Ԫ�й���Oԭ����ĿΪ9+4��![]() =11���ṹ��Ԫ��Siԭ����4�����ṹ��Ԫ���ϼ�Ϊ����-2����11+��+4����4=-6���ʶ��������ӵĻ�ѧʽͨʽΪ(Si4O11)n6n-����Ϊ��(Si4O11)n6n-��
=11���ṹ��Ԫ��Siԭ����4�����ṹ��Ԫ���ϼ�Ϊ����-2����11+��+4����4=-6���ʶ��������ӵĻ�ѧʽͨʽΪ(Si4O11)n6n-����Ϊ��(Si4O11)n6n-��


 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2 (g) �� 3 H2(g) ![]() 2NH3(g)��
2NH3(g)��
��1������Ӧijʱ��tʱ��n t (N2) = 13 mol��n t (NH3) = 6 mol����a =____mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������NH3�ĺ���(�������)Ϊ25%��ƽ��ʱNH3�����ʵ���_____��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȡ���ͬ����n(ʼ)��n(ƽ) =______��
��4��ԭ��������У�a��b =_____��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ���(N2)�æ�(H2)= ______��
��6��ƽ���������У�n(N2)��n(H2)��n(NH3) =______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й�����������Ӧ����������ȷ���ǣ� ��
��2H2S��SO2=3S����2H2O
��S��2H2SO4(Ũ)![]() 3SO2����2H2O
3SO2����2H2O
��3S��6KOH![]() 2K2S��K2SO3��3H2O
2K2S��K2SO3��3H2O
A.��Ӧ��˵��SO2�����ᷴӦ�����м��������������
B.��Ӧ�ڸ���ϡH2SO4�����ܷų�����
C.��Ӧ�ں͢�˵��S��������Ԫ�ص�����
D.��Ӧ��˵��S�Ⱦ����������־��л�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1.92gͭ����һ������Ũ���ᷴӦ����ͭ����ȫ����ʱ�ռ�������1.12L(��״����)����������������ʵ�����
A.0.12molB.0.09molC.0.11molD.0.08mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����32.64gͭ��140mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2��������ڱ�״���µ����Ϊ11.2L����ش�
(1)NO�����Ϊ________L��NO2�����Ϊ________L��
(2)������������ȫ���ͷź�����Һ�м���VmLamol/L��NaOH��Һ��ǡ��ʹ��Һ��Cu2��ȫ��ת���ɳ�������ԭ������Һ��Ũ��Ϊ__________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D����Ԫ�ص������������Ϊ1��4��5��7����˵������B��C��D��A�Ĵ���������֪Bԭ�ӵĴ���������Ϊ2��C��D��Aԭ�ӵĴ�����������Ϊ8��Aԭ�Ӻ����������������![]() ���Իش�
���Իش�
(1)��Ԫ�ط���Ϊ��A______B______C______D______
(2)д��B��C��D����������Ӧˮ����ķ���ʽ��______��______��______�����Ƚ�������ǿ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽������X(��������Ԫ�أ�Ħ������Ϊ270g��mol-1)����ɺ����ʣ�ijѧϰС�����������ʵ�飬�������嵥��A��ʹ�����ǵ�ľ����ȼ��
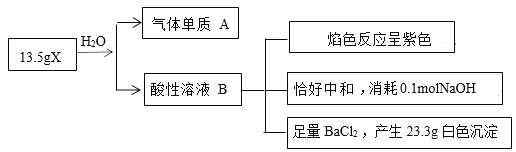
(1)X�����Ԫ��ΪO��______����Ԫ�ط��ű�ʾ����
(2)д��X��ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽ__________��
(3)X����������ǿ���ҶԻ����Ѻã������������������ڼ��������£�X����SO32-�����ӷ���ʽ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼����[La2(CO3)3]���������Ƹ�������Ѫ֢��ij��ѧС������ͼװ��ģ���Ʊ�̼���磬��ӦΪ2LaCl3+6NH4HCO3=La2(CO3)3��+6NH4Cl+3CO2��+3H2O������˵����ȷ���ǣ� ��
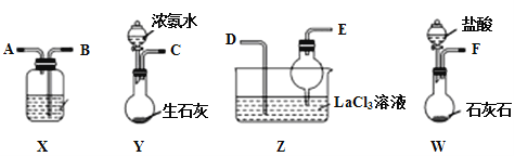
A.�������ҽӿڵ�����˳��F��B��A��D��E��C
B.װ��X��ʢ�ŵ��Լ�Ϊ����Na2CO3��Һ
C.װ��Z���ø���ܵ���ҪĿ��������Ӵ�������ӿ������ܽ�
D.ʵ�鿪ʼʱӦ�ȴ�Y�з�Һ©������ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ��b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӣ��ش��������⣺
(1)b��c��d�е�һ������������______![]() ��Ԫ�ط���
��Ԫ�ط���![]() ��
��
(2)a������Ԫ���γɵĶ�Ԫ���ۻ������У������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������______![]() �ѧʽ��д������
�ѧʽ��д������![]() ��
��
(3)��ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������______������������ṹ������______![]() �ѧʽ
�ѧʽ![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com