
ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ���������ʽ�������ľۺ�����̷���FeSO4��7H2O�����������£�
��1�������̢��в���������ͨ��������Һ�У���Һ����ɫ����________��
A��Ʒ����Һ B����ɫʯ����Һ
C������KMnO4��Һ D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��3�����̢��У�������������________��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����żܡ������ǣ�����Ҫ��������________________��
��5�����̢ݵ���pH��ѡ�������Լ��е�________����ѡ����ţ���
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70��80 �棬Ŀ����______________________��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣
���÷�����ƽ��ȡ2��700 g��Ʒ��
�ڽ���Ʒ�����������������������Ȼ�����Һ��
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ճ�������ʹ�õ����Ͻ��е��������ڵ������������ҵ�ϵ��������Ҫ���䴿�Ȳ��õ���98.2%������Ȼ�����������������Ϊ50%��70%��������ҪΪSiO2��Fe2O3��CaO��MgO��Na2O�ȡ���ҵ���������Ĺ�������ʾ��ͼ���£�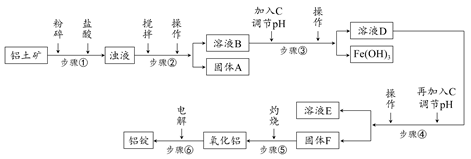
�� һЩ�������������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Mg(OH)2 |
| ��ʼ����pH(���ӳ�ʼŨ��0.01 mol/L) | 4 | 2.3 | 10.4 |
| ��ȫ����pH(����Ũ��<10��5 mol/L) | 5.2 | 4.1 | 12.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(FeC6H6O7)��һ�������յĸ�Ч���Ƽ��������̷�(FeSO4��7H2O)ͨ�����з�Ӧ�Ʊ���
FeSO4��Na2CO3=FeCO3��Na2SO4
FeCO3��C6H8O7=FeC6H6O7��CO2��H2O
�±��г�����ؽ������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0 mol��L��1����)��
| �������� | ��ʼ������pH | ������ȫ��pH |
| Fe3�� | 1.1 | 3.2 |
| Al3�� | 3.0 | 5.0 |
| Fe2�� | 5.8 | 8.8 |
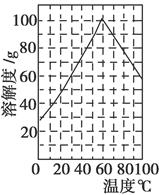
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ����dz�����ˮ������,��ˮFeCl3����������ҵ���Ʊ���ˮFeCl3��һ�ֹ�����ͼ��ʾ:
��1�����������������ռ�XӦ�� ������ĸ��ţ���
a.NaOH��Һ b.����ʳ��ˮ c.FeCl2��Һ d.����KI��Һ
��2��ȡ0.5 mL����FeCl3��Һ����50 mL��ˮ��,�ú��ɫ������������,������Ӧ�����ӷ���ʽΪ ��
��3��ʵ�����д�FeCl3��Һ�Ƶ�FeCl3��6H2O����Ĺ�����,���ȼ��� �ұ��ֹ���,Ȼ����еIJ�������Ϊ ����ȴ�ᾧ�����ˡ�
��4����H2S����ͨ��FeCl3��Һ�л���ֻ���,���䷴Ӧ�����ӷ���ʽΪ ��
��5������������ԭҺ�������һ�ֵͳɱ��Ĵ��ܵ��,��ؽṹ��ͼ��ʾ���缫����Ϊʯī��,����ԭ��Ϊ:Fe3++Cr2+ Fe2++Cr3+
Fe2++Cr3+
���طŵ�ʱ,Cl-������ ���������������;���ʱ,�����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С�齫һ����������·�徭Ũ�����ϡ���ᴦ����õ�һ�����Һ,���к���Cu2+��Fe2+��Fe3+��Al3+�Ƚ�������,��������������������Էֱ���ȡCuSO4��5H2O�����AlCl3
��Һ: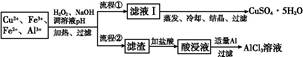
��֪:��ؽ������ӿ�ʼ��������ȫ����ʱ��pH��ΧΪ:
| ���� | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH��Χ | 2.2��3.2 | 5.5��9.0 | 4.1��5.0 | 5.3��6.6 |
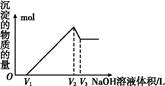
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͭ��һ���/����ͭ����������Բ��ϣ���ͨ�����з����Ʊ����ڲ����н�����ͭ�ù�����40%������ܽ⣬���Ƴ�CuF2��5HF��5H2O���ٽ���������������ڵIJ����У��ڸ���ķ�������������400%���м�����ˮ�����ͨ�뵪����
��1���Ʊ��������ò�������ò��������ԭ����______________________________________���û�ѧ����ʽ��ʾ����
��2�� �/����ͭ��طŵ�ʱ�ܷ�ӦΪ�û���Ӧ���仯ѧ����ʽΪ____________________________________________________��
��3������ȡ�����Ʊ�����Ʒ������CuF2��CuO�� 2.120 g����ͨ����м���ϡ��������ȫ�ܽ⣬Ȼ���������������������Һ���ó�����������������գ���1.680 g��ɫ���壬������Ʒ��CuF2��CuO�����ʵ���֮�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ի�ͭ��(��Ҫ�ɷ���CuFeS2,����������SiO2)Ϊԭ����ͭ�ķ�����Ϊ������ͭ��ʪ����ͭ���֡�������,ʪ����ͭ�����½�չ,��ѧ�ҷ�����һ��ϸ��������ˮ��Һ������������,���Խ���ͭ��������������:4CuFeS2+2H2SO4+17O2 4CuSO4+2Fe2(SO4)3+2H2O��ij�������ø�ԭ������ͭ���̷��Ĺ�������:
4CuSO4+2Fe2(SO4)3+2H2O��ij�������ø�ԭ������ͭ���̷��Ĺ�������: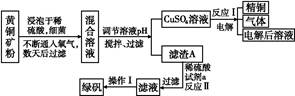
�ش���������:
(1)���������п����������������е�����ҺpH������������(�����)��
| A��Cu�� | B��Cu2(OH)2CO3�� | C��H2SO4�� | D��Fe��E.CuO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�������Թ�ҵ��ˮ�к���һ������Na����Al3���� Fe3����Cu2����Cl�����ó�������ͼ��ʾ�Ĺ�������ͼ�����ó���������������ᡢ���ҵ�����еķ���м���ӷ�ˮ�����������Ȼ�������������NaCl����ͽ���ͭ�������˺ܺõ���ᾭ��Ч�档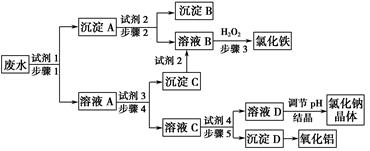
����д���пհף�
(1)ͼ���Լ�1��________���Լ�2��________��
(2)����1�Ͳ���2���õ��IJ���������________��
(3)����1��Ӧ�����ӷ���ʽΪ_______________________________________��
(4)����3��Ӧ�����ӷ���ʽΪ_______________________________________��
(5)�ӽ�ԼҩƷ�ͻ������濼�ǣ�����5��������Ӧ�����ӷ���ʽӦΪ__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����֣���Ҫ�ɷ�ΪFe����Ʒ�к�������ͭ�ȣ������ɷֺ��ԣ�,Ϊ�˲ⶨ�úϽ������ĺ���,����������¹������̣�
��1����ҺC�����ʺ���_ _��д��ѧʽ����
��2�������֤��ҺA�к�Fe2+��������Fe3+____ _____��
��3��������Fe2O3�������������Ϊb g���������Ʒ����Ԫ�����������ı���ʽΪ���ú�a��b��ʽ�ӱ�ʾ��___ __��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com