
����ͭ��һ���/����ͭ����������Բ��ϣ���ͨ�����з����Ʊ����ڲ����н�����ͭ�ù�����40%������ܽ⣬���Ƴ�CuF2��5HF��5H2O���ٽ���������������ڵIJ����У��ڸ���ķ�������������400%���м�����ˮ�����ͨ�뵪����
��1���Ʊ��������ò�������ò��������ԭ����______________________________________���û�ѧ����ʽ��ʾ����
��2�� �/����ͭ��طŵ�ʱ�ܷ�ӦΪ�û���Ӧ���仯ѧ����ʽΪ____________________________________________________��
��3������ȡ�����Ʊ�����Ʒ������CuF2��CuO�� 2.120 g����ͨ����м���ϡ��������ȫ�ܽ⣬Ȼ���������������������Һ���ó�����������������գ���1.680 g��ɫ���壬������Ʒ��CuF2��CuO�����ʵ���֮�ȡ�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������(CH3OCH3)����ɫ���壬����Ϊһ��������Դ���ɺϳ���(���ΪH2��CO��������CO2)ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
(��)CO(g)��2H2(g)=CH3OH(g) ��H1����90.1 kJ��mol��1
(��)CO2(g)��3H2(g)=CH3OH(g)��H2O(g) ��H2����49.0 kJ��mol��1
ˮú���任��Ӧ��
(��)CO(g)��H2O(g)=CO2(g)��H2(g) ��H3����41.1 kJ��mol��1
�����Ѻϳɷ�Ӧ��
(��)2CH3OH(g)=CH3OCH3(g)��H2O(g) ��H4����24.5 kJ��mol��1
�ش��������⣺
(1)Al2O3�Ǻϳ���ֱ���Ʊ������ѷ�Ӧ��������Ҫ�ɷ�֮һ����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ����������____________________________________________(�Ի�ѧ����ʽ��ʾ)��
(2)���������Ѻϳɷ�Ӧ(��)����COת���ʵ�Ӱ��
________________________________________________________________________��
(3)���о����ڴ���(��CuZnAlO��Al2O3)��ѹǿΪ5.0 MPa�������£���H2��COֱ���Ʊ������ѣ������ͼ��ʾ������COת�������¶����߶����͵�ԭ����_________________________________________________��
(4)������ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߵ��ŵ㣬�������ܶȸ��ڼ״�ֱ��ȼ�ϵ��(5.93 kW��h��kg��1)���������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦΪ__________
_____________________��һ�������ѷ��Ӿ����绯ѧ���������Բ���________________�����ӵĵ������õ�ص����������ѹΪ1.20 V�������ܶ�E��_______________(��ʽ���㡣�����ܶȣ�����������/ȼ��������1 kW��h��3.6��106 J)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������������(��Ҫ�ɷ�ΪFe2O3��FeO��SiO2��)Ϊԭ���Ʊ��ߵ���������(Fe2O3)�����������������£�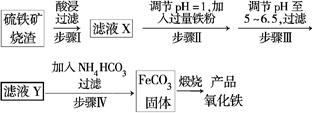
�Իش��������⣺
(1)��ҺX�к��еĽ�����������______________(�����ӷ���)��
(2)������п�ѡ��________������Һ��pH(����ĸ)��
| A��ϡ���� | B����ˮ | C������������Һ | D�����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ���������ʽ�������ľۺ�����̷���FeSO4��7H2O�����������£�
��1�������̢��в���������ͨ��������Һ�У���Һ����ɫ����________��
A��Ʒ����Һ B����ɫʯ����Һ
C������KMnO4��Һ D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��3�����̢��У�������������________��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����żܡ������ǣ�����Ҫ��������________________��
��5�����̢ݵ���pH��ѡ�������Լ��е�________����ѡ����ţ���
A��ϡ���� B��CaCO3 C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70��80 �棬Ŀ����______________________��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣
���÷�����ƽ��ȡ2��700 g��Ʒ��
�ڽ���Ʒ�����������������������Ȼ�����Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��G�����ʼ�Ĺ�ϵ��ͼ������B��DΪ��̬���ʡ�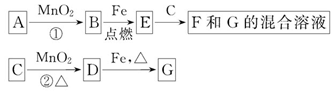
��ش��������⡣
��1������C��E�����Ʒֱ�Ϊ_______��_______��
��2����ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽΪ_________����ֻ���ڼ�������½��У���Ӧ��AӦΪ______________��
��3��MnO2�ڷ�Ӧ�ٺͷ�Ӧ���е����÷ֱ���_______��_______��
��4�������Ƶ�F��ҺӦ����_______�Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ���_______��ʵ������Ϊ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС�鰴���������̽��С���þ���Ͻ��Ʊ����������塱��ʵ�顣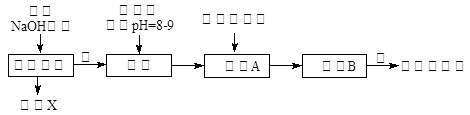
��1��þ���Ͻ��м�NaOH��Һ�����ӷ�Ӧ����ʽΪ ����������X��ԭ�ӽṹʾ��ͼ ������A�Ļ�ѧʽΪ ��
��2��д����������ˮ�еĵ��뷽��ʽ �������ڰ����IJ���������Ũ���� �����ˡ����
��3������ȤС��Ϊ�ⶨþ���Ͻ��и���ɵ����������������ͼװ�ã�����Ҫ�ⶨ�������� ���� �� 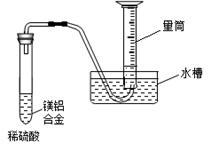
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�Dzⶨ���ۣ���þ�ۣ��Ĵ��ȵ�ʵ��װ�á����õ�NaOH�������������ʵ���Ũ��Ϊ4.5 mol��L��1����ͬʱ�������ƽ�Ķ������±���ʾ��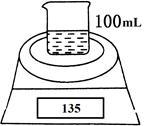
| ʵ����� | ʱ��/min | ������ƽ�Ķ���/g |
| �ձ���NaOH��Һ | 0 | 120 |
| �ձ���NaOH��Һ����Ʒ | 0 | 135 |
| 1 | 134.5 | |
| 2 | 134.1 | |
| 3 | 133.8 | |
| 4 | 133.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС�����÷�������CuO��Al2O3��SiO2����ģ�ҵ����ȡ���Ĺ��գ��������ͼ��ʾ�ļ������̣�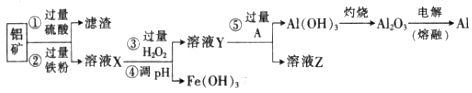
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | 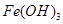 | 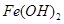 |  |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
 ʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����___________�����������ƣ���
ʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����___________�����������ƣ���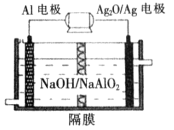
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com