
�ö������ȣ�ClO2���������ƣ�Na2FeO4Ħ������Ϊ166g/mol�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl����Fe3����
��1������Ե�λ���������������õ��ĵ���������ʾ����Ч�ʣ���ô��ClO2��Na2FeO4��Cl2��������ɱ����������Ч���ɴ�С��˳����________��__________��__________��
��2��Na2FeO4����ˮ��ų�һ����ɫ��ζ���壬��ɱ������������ˮ�е��������ʵ�ԭ���������ӷ���ʽ��ʾΪ_______________��
��3����ҵ��CH3OH��NaClO3Ϊԭ����������������ȡClO2��ͬʱ����CO2���壬��֪�÷�Ӧ��Ϊ�������У���һ��Ϊ2ClO3-��2Cl����4H����2ClO2����Cl2����2H2O��
��д���ڶ�����Ӧ�����ӷ���ʽ______________��
�ڹ�ҵ����ʱ���ڷ�Ӧ���м�����Cl������������________________��
�������лᷢ������ӦClO3-��Cl����H����Cl2����H2O��δ��ƽ��������÷�Ӧ��Ļ��������Cl2���������Ϊ3��73������ʼͶ��ʱCH3OH��NaClO3�����ʵ���֮��Ϊ____________��
��4����֪����������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ��ClO2Ҳ���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ�����60��ʱ��Ӧ�Ƶá�ijѧ������ͼ��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�á����ʣ�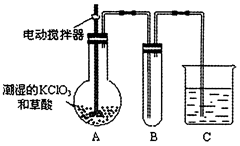
��A���ֻ�Ӧ�����¶ȿ��ƣ���ˮԡ���ȣ�װ�ã�B���ֻ�Ӧ����ʲôװ��___________��
��C��Ӧװ���Լ�Ϊ____________��C�з�����Ӧ�Ļ�ѧ����ʽΪ��________________��
��1��ClO2; Cl2 Na2FeO4 ��2�� 4FeO42-+10 H2O=4Fe��OH��3+8OH-+3O2����3�� ��CH3OH+3Cl2+H2O=6Cl-+ CO2+6H+.�ڴ�����1:6.1��4���٣���ˮ��������NaOH��Һ 2ClO2+2NaOH=NaClO2+NaClO3+H2O
���������������1�� ClO2��Na2FeO4��Cl2��������ɱ����������Ч�ʷֱ���ClO2: 67.5g��5mol=" 13.5g/mol;" Na2FeO4: 166g��3mol=55.33g/mol;Cl2:71g/ 2mol=35.5g/mol.������������ɱ����������Ч���ɴ�С��˳����ClO2> Cl2>Na2FeO4. ��2�� ��������ɵ�Na2FeO4��H2O����ΪO2������ɱ������������������ԭΪFe3+��Fe3+ˮ�����Fe��OH��3��������ˮ�е��������ʡ��Ӷ�������ˮ���÷�Ӧ�����ӷ���ʽΪ��4FeO42-+10 H2O=4Fe��OH��3+8OH-+3O2������3���ٵڶ�����Ӧ�����ӷ���ʽΪ��CH3OH+3Cl2+H2O=6Cl-+ CO2+6H+�����ɢ��е�������Ӧ����ʽ���Կ�����ת����ͬ�ĵ��ӣ�Cl-���������������ȡ�֮�����ڹ�ҵ����ʱҪ�ڷ�Ӧ���м�����Cl��������ΪCl-�����������á��۽���һ����ڶ����ķ���ʽ���ӿɵ��ܷ���ʽ��6ClO3-��CH3OH��6H��=CO2��+6ClO2����5H2O��������ӦClO3-��Cl����H����Cl2����H2O��ƽ�ɵã�ClO3-��5Cl����6H��=3Cl2����3H2O����÷�Ӧ��Ļ��������Cl2���������Ϊ3��73����������������ʵ���Ϊ73mol����Cl2Ϊ3mol��CO2��ClO2�����ʵ���Ϊ70mol������3mol��Cl2����NaClO3�����ʵ���1mol�����ݷ���ʽ6ClO3-��CH3OH��6H��=CO2��+6ClO2����5H2O��֪����CO2��ClO2�����ʵ���Ϊ70mol����CH3OH�����ʵ���Ϊ10mol������ NaClO3�����ʵ���Ϊ60mol�����Թ�����CH3OH�����ʵ���Ϊ10mol������ NaClO3�����ʵ���Ϊ60mol+3mol=61mol�������ʼͶ��ʱCH3OH��NaClO3�����ʵ���֮��Ϊ10:61=1:6.1.��4�� ����ΪClO2�ķе�ͣ����ӷ�������B���ֻ�Ӧ�����װ��������װ�á������ñ�ˮ���н��¡���CΪβ������װ�ã�����ClO2��������Ӧ������ͨ����NaOH��Һ�����ա���Ӧ�ķ���ʽΪ��2ClO2+2NaOH=NaClO2+NaClO3+H2O��
���㣺��������ˮ�IJ�ͬ������������Ч�ʵıȽϡ�ClO2���Ʒ������ʼ��漰�Ļ�ѧ��Ӧԭ������Ҫ����������ԭ��Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ����ƽ����д��ʵ��װ�õ�ѡ��Ӧ�ú��йؼ����֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)(4��)д������������ˮ��Һ�еĵ��뷽��ʽ��
HNO3 Ba(OH)2
NaHCO3 NaHSO4
(2)(4��)��ʵ�����Ʊ������Ĺ����У����������ͨ����������������Һ�������գ���д���˷�Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ��
�û�ѧ��Ӧ�У��������� ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������A��B��C��D��E�����ǵ������ӿ�����Na����NH4+��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����NO3-��SO42-��CO32-����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⣺
��1���������У�һ��û�е���������____________��������������ͬ�������εĻ�ѧʽ�� ��
��2��D�Ļ�ѧʽΪ_________��D��Һ�Լ��Ե�ԭ����(�����ӷ���ʽ��ʾ)��
��
��3��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��___________________________________ _____��
��4�����ʵ�����B�������������ӣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��10�֣���ҵ���Ի�����Ϊԭ������������Ҫ��Ϊ�����ν��У������ա������������ա���ش����и����⣺
��1�����ջ������γɵ�¯�����뾭������ϴ�ӡ��������� �����豸���ƣ�
��2���������η�Ӧ2SO2(g)+O2(g)  2SO3(g)����H��0��550 ��ʱ��SO2ת��ΪSO3��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ��ͼ��ʾ��
2SO3(g)����H��0��550 ��ʱ��SO2ת��ΪSO3��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ��ͼ��ʾ��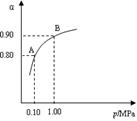
������Ҫ���SO2��ת���ʣ���Ӧ������Ӧ�� �� ����ѹ����ߡ������͡������� ������ͨ������¹�ҵ�����в��ó�ѹ��ԭ���� ��
��2.0 mol SO2��1.0 mol O2����5 ���ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10 M Pa���÷�Ӧ��ƽ�ⳣ������ ��
��3��Ϊѭ�����ô�����������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ���֪�Ϸ������к���V2O5��VOSO4�������Բ�������������֪��VOSO4������ˮ��V2O5������ˮ��NH4VO3������ˮ���ù��յ���������ͼ��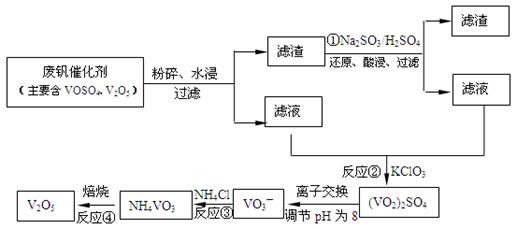
��Ӧ�٢ڢۢ�������������ԭ��Ӧ���� ����������ţ�����Ӧ�۵����ӷ���ʽΪ ���ù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ�������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ��(NH4Cl������������Һ��V2O5��������)���¶ȡ�������ͼ�Խ�������Ȼ��ϵ�����¶ȣ� �� �� 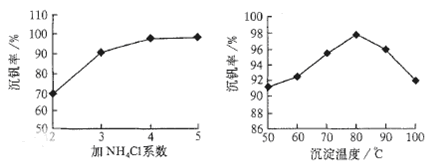
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij�ᾧˮ���ﺬ�����������Ӻ�һ�������ӡ���ȡ����������Ϊ45.3 g�ĸýᾧˮ����ֱ��Ƴ���Һ��������һ����μ���NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����������ݳ����������д̼�����ζ����ʹʪ��ĺ�ɫʯ����ֽ���������Ⱥƿ��ռ���2.24 L������(��״��)������ɫ�������ٲ�������ʧ��
��һ����μ���Ba(OH)2��Һ����ʼ�������ƣ����������а�ɫ���������ˣ���ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����46.6 g��
��ش��������⣺
��1����ͨ������ȷ���ýᾧˮ����Ļ�ѧʽΪ ��
��2����������������Һ�м����Ba(OH)2��Һ�����ʵ���Ũ��Ϊ2.0 mol��L-1��
�ټ�������Ba(OH)2��Һ����Ӧ�����ӷ���ʽΪ ��
�ڼ���һ������Ba(OH)2��Һ�������ó����������ʵ��������Ӧ�����ӷ���ʽΪ ��
��������75 mL��Ba(OH)2��Һ����õ��ij�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������������ԭ��ӦҲ�����ȷ�Ӧ����( )
| A�����ȵ�̿�������̼��Ӧ | B������ϡ����ķ�Ӧ |
| C��������������ķ�ĩ���Ȼ�茶����� | D��������Ʒ����ķ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������һ��Ũ�ȵ�����ʱ��������Ӧ�����ӷ���ʽΪ��
aFe+bNO3-+cH+�TdFe2++fFe3++gNO��+hNO2��+kH20�������й��ƶ��У�����ȷ���ǣ�������
| A��2d+3f=3g+h |
| B��c+b=2d+3f |
| C��HNO3��������ǿ��Fe3+ |
| D����Ӧ��ÿ����5��6gFe��ת��0��2mol��0��3mole- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪����������Һ�пɷ��������������ӷ�Ӧ��Ge4����Fe2��===Fe3����Ge3���� Sn2����2Fe3��===2Fe2����Sn4�����ɴ˿���ȷ��Fe2����Ge3����Sn2���������ӵĻ�ԭ����ǿ������˳����
| A��Sn2����Fe2����Ge3�� | B��Sn2����Ge3����Fe2�� |
| C��Ge3����Fe2����Sn2�� | D��Fe2����Sn2����Ge3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij��Ӧ�ķ�Ӧ�����������У�K2Cr2O7��KCl��CrCl3��Cl2��HCl��H2O����֪��Ӧ�����з������±仯��K2Cr2O7��CrCl3��������˵������ȷ����
| A���ɴ˷�Ӧ��֪������K2Cr2O7��Cl2 |
| B���������ͻ�ԭ�������ʵ���֮��Ϊ1:6 |
| C����ת��0.2mol����ʱ�����ɵĻ�ԭ��������ʵ���Ϊ0.1mol |
| D������������ԭ��Ӧ����Ԫ����μӷ�Ӧ����Ԫ�صı�Ϊ3:7 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com