
| m |
| M |
| m |
| M |
| m |
| M |
. |
| M |
| m(��) |
| n(��) |
| ||
| m |
| M |
| 1g |
| 18g/mol |
| 1 |
| 18 |
| 1 |
| 18 |
| 1 |
| 9 |
| 1 |
| 9 |
| n | ||
|
| m |
| M |
| 1 |
| 16 |
| 4 |
| 32 |
| 32 |
| 64 |
| 60g |
| 64g/mol |
| 15 |
| 16 |
| 40g |
| 32g/mol |
| 20 |
| 16 |
| 15 |
| 16 |
| 20 |
| 16 |
| 100 | ||||
|
| 45.71 |
| 32 |
| 10 |
| 7 |
| 10 |
| 7 |
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| X | Y | |
| Z | W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
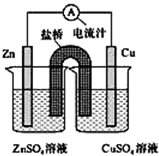 ��ͼΪһԭ��ص�װ��ʾ��ͼ������˵������ȷ���ǣ�������
��ͼΪһԭ��ص�װ��ʾ��ͼ������˵������ȷ���ǣ�������| A��ԭ��ع���ʱ���ܷ�ӦΪ��Zn+Cu2+=Zn2++Cu |
| B��ԭ��ع���ʱ��������ͭ����������������п�� |
| C��ԭ��ع���ʱ��������K+����CuSO4��Һ |
| D�������Cu�缫��Zn�缫������������ָ�뷴��ƫת |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��FeCl3��Һ�ܹ����磬����FeCl3��Һ�ǵ���� |
| B��CO2ˮ��Һ�ܹ����磬����CO2�ǵ���� |
| C��Һ̬��ͭ�����Ժܺã�����ͭ�ǵ���� |
| D��NaOH��������ˮ���ܵ��磬����NaOH�ǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��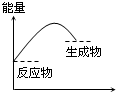 ��Ӧ���� |
B��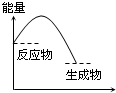 ��Ӧ���� |
C��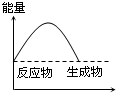 ��Ӧ���� |
D��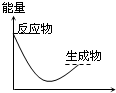 ��Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.5mol�������к���C=C˫����Ϊ1.5NA | ||
| B��2.8g��ϩ�ͱ�ϩ�Ļ������������̼ԭ����Ϊ0.2NA | ||
C����״���£�1L�״���ȫȼ�պ����ɵ�CO2������ĿԼΪ
| ||
| D��1 mol����-CH3�������ĵ�������Ϊ8 NA |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com