
����Ŀ������ý(V2O5)�Ǵ����������õĴ�����Ϊ�ۺ����ã�������Ա����������һ�����ӽ��������շϷ����¹��գ������ʴ�90%���ϡ���֪�Ϸ������к���V2O5��VOSO4�������Բ������������Ͽ�֪��VOSO4������ˮ��V2O5������ˮ��NH4VO3������ˮ���ù��յ���������ͼ��

��1��ˮ��ʱ��Ϊ����߷Ϸ��Ľ����ʣ����˷��飬�����Բ�ȡ�Ĵ�ʩ��___________________________________��__________________________________��д��2������
��2��д����Ӧ�ٵ����ӷ���ʽ_______________________________________________��
��3���ù����з�Ӧ�۵ij������ǻ��շ��Ĺؼ��������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ����NH4Cl������������Һ��V2O5�������ȣ����¶ȡ�������ͼ��������ʵ��Ȼ��ϵ�����¶ȣ��Ȼ��ϵ��Ϊ___________���¶�Ϊ_____________��

��4����Ӧ�ڷ�������Һ�е���Ԫ����Cl������ʽ���ڣ���д����Ӧ�ڵĻ�ѧ����ʽ__________________________________________________________________��
��5���������ϵ�֪��NH4VO3Ҳ��ƫ����泥�����Է�����Ϊ117��20��ʱ��NH4VO3�ܽ��Ϊ0.468g����֪���ӽ�������Һ��c��VO3����=0.10mol/L����Ӧ�۳���ʱ����������0.10mol/L��NH4Cl��Һ��ͨ����ʽ�����жϴ�ʱ�Ƿ���NH4VO3��������������Һ����仯���Բ��ƣ�____________
��6��д�������շ�Ӧ��������NH4VO3�Ʊ�V2O5�Ļ�ѧ����ʽ__________________��
���𰸡� ���� �ʵ������¶ȣ��ӳ�����ʱ�� V2O5+SO32-+4H+=2VO2++SO42-+2H2O 4 80�� 6VOSO4+KClO3+3H2O=3(VO2)2SO4+KCl+3H2SO4 ��20��ʱNH4VO3�ı�����Һ����c(NH4+)=c(VO3-)=0.468��10/117=0.04mol/L��Ksp=1.60��10-3 ,��c(NH4+)��c(VO3-)=(0.10/2)��(0.10/2)=2.50��10-3>Ksp=1.60��10-3 ,���г������� 2NH4VO3 ![]() 2NH3+V2O5+H2O
2NH3+V2O5+H2O
��������(1) ˮ��ʱ��Ϊ����߷Ϸ��Ľ����ʣ����˷��飬�����Բ�ȡ�Ĵ�ʩ���衢�ʵ������¶ȡ��ӳ�����ʱ��ȴ�ʩ��
(2) �����⣬��Ӧ����V2O5����������SO32-����ԭ������Ӧ�����ӷ���ʽΪV2O5+SO32-+4H+=2VO2++SO42-+2H2O��
(3)����ͼ������ɵã�Ҫʹ��������ߣ��ɿ����Ȼ��ϵ��Ϊ4���¶���80�棻
(4) �Ϸ��п���������ΪVOSO4�����Է�Ӧ�ڵĻ�ѧ����ʽΪ6VOSO4+KClO3+ 3H2O= 3(VO2)2SO4+KCl+3H2SO4 ��
(5) �����ܽ�ȵĶ���ɵã���20��ʱNH4VO3�ı�����Һ����c(NH4+)=c(VO3-)= ![]() 0.04mol/L����Ksp(NH4VO3)= (0.04mol/L)2=1.60��10-3 mol2/L2������ʱ��Һ��KQ=c(NH4+)��c(VO3-)=(0.10/2)��(0.10/2)=2.50��10-3 >Ksp(NH4VO3)=1.60��10-3�����г���������
0.04mol/L����Ksp(NH4VO3)= (0.04mol/L)2=1.60��10-3 mol2/L2������ʱ��Һ��KQ=c(NH4+)��c(VO3-)=(0.10/2)��(0.10/2)=2.50��10-3 >Ksp(NH4VO3)=1.60��10-3�����г���������
(6) �����շ�Ӧ��������NH4VO3�Ʊ�V2O5�Ļ�ѧ����ʽΪ2NH4VO3 ![]() 2NH3+ V2O5+ H2O��
2NH3+ V2O5+ H2O��


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ױ�����������ܷ����ķ�Ӧ��
A. ȡ����Ӧ B. �ӳɷ�Ӧ C. ˮ�ⷴӦ D. ��ȥ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ���Ϊ�������ʡ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ��
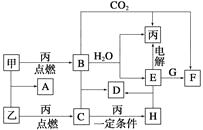
��ش��������⣺
��1���û�ѧʽ��ʾ����Ϊ__________��HΪ__________��
��2��A�ĵ���ʽΪ___________________________________________________��
��3�����E��ˮ��Һʱ��E��������_______________________________��
��4��д��B��C����D�Ļ�ѧ����ʽ��__________________________________��
д��E��G����F�����ӷ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ú�������ǽ���ת��Ϊ��ȼ������Ĺ��̣���Ҫ��ѧ��Ӧ����ʽΪ__________��úҲ����ֱ��Һ��������________________�����ѧ�仯���������仯������
��2������ϩ�ϳɾ���ϩ�Ļ�ѧ����ʽΪ_______________�ø߷��ӻ����������Ϊ___�����ø߷��ӻ��������Է�������Ϊ42 000������ۺ϶�Ϊ__��
��3��һ�ּ���ˮ���ᄃ���У�ƽ��ÿ46��ˮ���ӹ���8����������ÿ��������������1��������ӻ�ˮ���ӣ�����8������������6�����ɵ��Ǽ�����ӣ�����2����ˮ������䣬���ֿ�ȼ����ƽ����ɿɱ�ʾΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�ˮ��Һ�ܵ��磬�����ڷǵ���ʵ����� ��
A. CH3CH2COOH B. SO2 C. NH4HCO3 D. Cl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���ܹ���������̼����������� ��
A. ������չ�������� B. ��ʹ����Ȼ����ȼ��
C. ���Խ�ֹ�ŷŶ�����̼ D. ��������Դ�����ٶԻ�ʯȼ�ϵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ҫ��һԪ�ᣬ���л�������Ӧ�ж���Ӧ�á�����25 ��ʱ��pH��3�Ĵ��ᡣ��ش��������⣺
��1����������м������������ƹ��壬��ʱ��Һ��![]() ________(��������������С������������)��
________(��������������������������)��
��2����������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������Һ��pH________(����>����<����������)7�������ӷ���ʽ��ʾ��ԭ��_____________________________________��
��3����������м���pH��11��NaOH��Һ���Ҷ��ߵ������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����___________________________________________��
��4����������м���NaOH��Һ����Һǡ�ó����ԣ���ʱc(Na��)______c(CH3COO-)(����>������<����������)��
��5����������м���һ����NaOH��Һ�����û��ҺpH��6�������Һ��c(CH3COO-)��C(Na��)��________mol/L(��дȷ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���о��к���ɫ����������Աȷ������ý��۲���ȷ���ǣ�������
|
|
|
�� | �� | �� |
A. �����еĺ���ɫ���壬�ƶϲ���������һ���ǻ������
B. ����ɫ���岻�ܱ�������ľ̿��Ũ��������˷�Ӧ
C. ����˵��Ũ������лӷ��ԣ����ɵĺ���ɫ����Ϊ��ԭ����
D. ������������м���CO2���ɴ�˵��ľ̿һ����Ũ���ᷢ���˷�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����14�֣�̼������������ѧ��ѧ��Ҫ�ķǽ���Ԫ�أ��ڹ�ũҵ�������й㷺��Ӧ�á�
(1�����ڷ������칬һ�����ij������Ż����ȼ����Һ̬ƫ�����£�CH3��2N��NH2����������Һ̬�����������������ڷ�Ӧ�����зų�����������ͬʱ������������Ⱦ�����塣��֪�����£�1 gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
��2��298 Kʱ����2L���ܱ������У��������淴Ӧ��
2NO2(g)![]() N2O4(g) ��H����a kJ��mol��1(a��0)
N2O4(g) ��H����a kJ��mol��1(a��0)
N2O4�����ʵ���Ũ����ʱ��仯��ͼ����ƽ��ʱ��N2O4��Ũ��ΪNO2��2�����ش��������⡣

��298kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ________��
�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����

a��A��C����ķ�Ӧ���ʣ�A��C
b��B��C����������ƽ����Է���������B��C
c��A��C�����������ɫ��A�Cdz
d����״̬B��״̬A�������ü��ȵķ���
������Ӧ��398K���У�ijʱ�̲��n(NO2��="0.6" mol n(N2O4��=1.2mol�����ʱV������ V���棩������>������<������=������
��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
�Է���ͼ��a��b��c��d��e����㣬
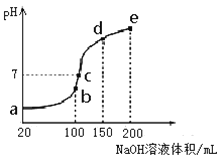
��ˮ�ĵ���̶�������__________��
������Һ��c(OH-)����ֵ��ӽ�NH3��H2O�ĵ��볣��K��ֵ���� ��
����c�㣬��Һ�и�����Ũ���ɴ�С������˳����_______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com